Cardiovascular System
Blood Vessel - Metaplasia
Narrative
Metaplasia is the transformation of one differentiated cell type to another differentiated cell type. Metaplasia of the vascular system is primarily associated with large arteries and is seen as either cartilaginous or osseous forms (Figure 1, Figure 2, Figure 3, and Figure 4). Metaplasia occurs rarely as a spontaneous lesion but, rather, as part of the progression of mineralization or atherosclerotic lesions. Typically, as vascular inflammation progresses, vessels may undergo mineralization, leading to cartilage formation and ultimately osseous metaplasia. Bony lesions may be composed of mature well-differentiated cells and contain marrow elements. In toxicity and carcinogenicity studies, this is generally considered a background lesion and is not typically exposure related.
The fact that cartilaginous metaplasia is most often localized to the aortic region is consistent with the hypothesis that mechanical stress is an important factor in promoting vascular metaplasia. However, a genetic predisposition for cartilaginous metaplasia of the arteries has been identified in C57BL/6J mice. These lesions usually occur in the aortic root and progress to calcification. Widespread cartilaginous metaplasia involving the aortic arch, as well as the aortic root, has also been seen in apoE-knockout mice. In Smad6-knockout mice, cartilaginous metaplasia and trabecular bone formation containing marrow elements have been noted in the aorta and around the outflow track of the heart.
Whenever present, metaplasia of blood vessels should be diagnosed but should not be graded. The site should be the affected organ, and the specific vessel affected (e.g., artery or vein) should be included as a site modifier. If the type of blood vessel cannot be determined, the site modifier "blood vessel" may be used. Lesions in protocol-required great vessels, such as aorta, should be recorded with the blood vessel as the site (e.g., Aorta - Metaplasia, Osseous). The type of metaplasia (e.g., cartilaginous, osseous) should be included in the diagnosis as a qualifier.
Mathieu P, Roussel JC, Dagenais F, Aneon I. 2003. Cartilaginous metaplasia and calcification in aortic allograft is associated with transforming growth factor β1 expression. Thorac Cardiovasc Surg 126:1449-1454.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/14666018Maxie MG, Robinson WF. 2007. Cardiovascular system. In: Jubb, Kennedy and Palmer's Pathology of Domestic Animals, Vol 3, 5th ed (Maxie MG, ed). Saunders Elsevier, Edinburgh, 57-58.
Nguyen N, Naik V, Speer MY. 2012. Diabetes mellitus accelerates cartilaginous metaplasia and calcification in atherosclerotic vessels of LDLr mutant mice. Cardiovasc Pathol 22(2):167-175.
Full Text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3481013/Qiao J-H, Fishbein MC, Demer LL, Lusis AJ. 1995. Genetic determination of cartilaginous metaplasia in mouse aorta. Arterioscler Throm Vasc Biol 15:2265-2272.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/7489252Rattazzi M, Bennett BJ, Bea F, Kirk EA, Ricks JL, Speer M, Schwartz SM, Giachelli CM, Rosenfeld ME. 2005. Calcification of advanced atherosclerotic lesions in the innominate arteries of ApoE-deficient mice: Potential role of chondrocyte-like cells. Atheroscler Thromb Vasc Biol 25(7):1420-1425.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/15845913Rosenfeld ME, Polinsky P, Virmani R, Kauser K, Rubanyi G, Schwartz SM. 2000. Advanced atherosclerotic lesions in the innominate artery of the apoE knockout mouse. Arterioscler Thromb Vasc Biol 20(12):2587-2592.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/11116057Wallin R, Wajih N, Grenwood GT, Sane DC. 2001. Arterial calcification: A review of mechanisms, animal models, and the prospects for therapy. Med Res Rev 21(4):274-301.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/11410932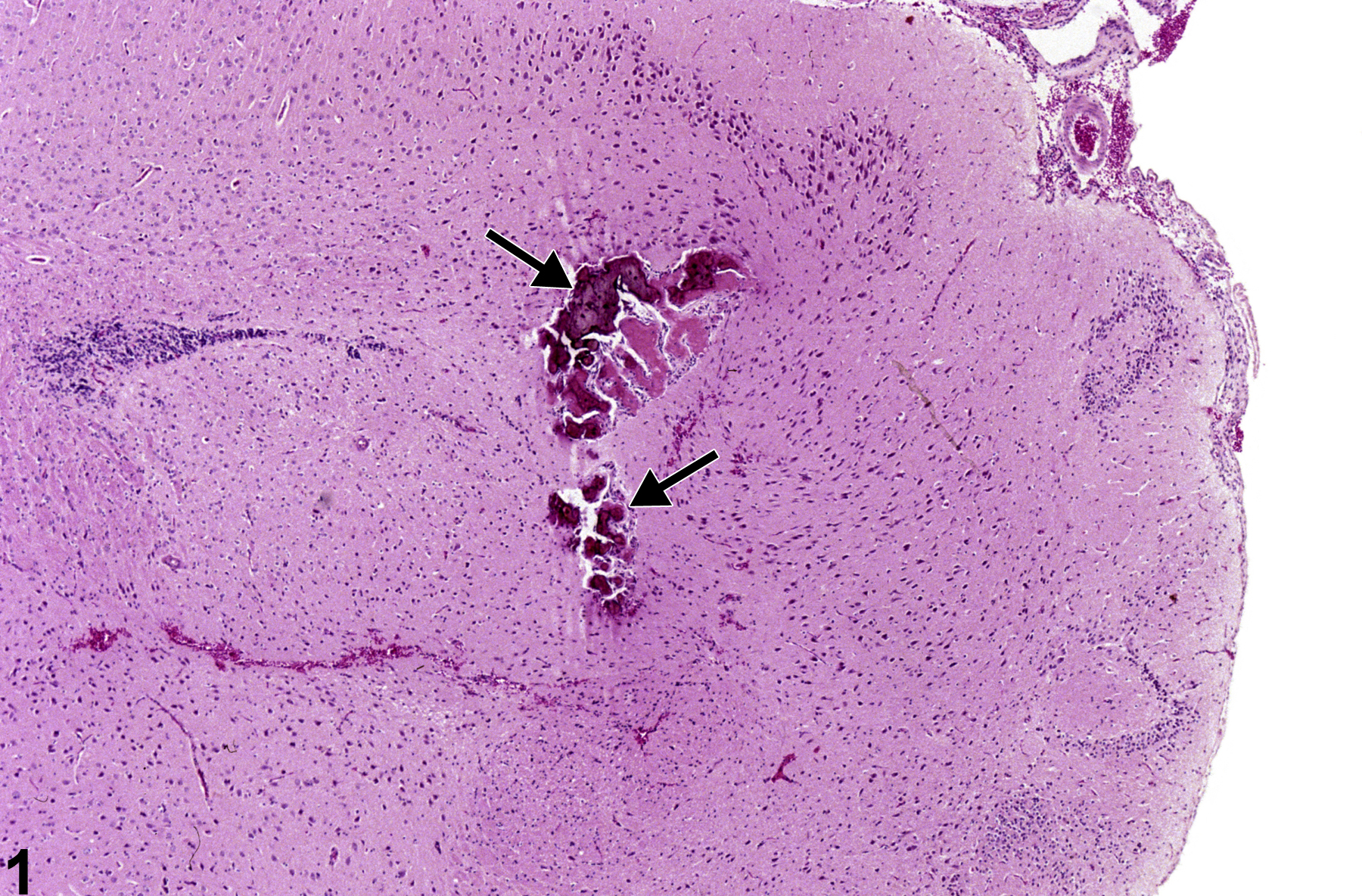
Brain, Artery - Metaplasia, Osseous in an F344/N male rat from a chronic study. There is deeply basophilic bone (arrows) in a vessel.





