Musculoskeletal System
Skeletal Muscle - Fibrosis
Narrative
Fibrosis is the end result of a cascade of events that begins with tissue injury and inflammation and ends in permanent scar formation. When tissue is damaged, profibrotic cytokines, such as transforming growth factor beta, are released by the infiltrating inflammatory cells. These cytokines signal the fibroblasts to migrate into the affected region and to begin producing and remodeling the extracellular matrix. The stromal fibroblasts then begin producing cytokines, growth factors, and proteases that further trigger and uphold the inflammatory/profibrotic conditions. While fibroblasts are fundamental for maintaining tissue homeostasis and regulating normal wound repair, they also serve as a crucial intermediate in the formation of chronic fibrotic diseases. During conditions of chronic or extensive muscle damage, persistent inflammation, prolonged fibroblastic activation, and attenuated reparative capacity of the satellite cells ultimately lead to excessive accumulation of extracellular matrix components. This inhibits myogenic repair and leads to the replacement of muscle with fibrotic/scar tissue.
While nutritional, exertional, and toxic myopathies can result in extensive regions of muscle damage, repair is often extremely effective, and thus fibrosis is minimal to absent. In contrast, the reparative process is often ineffective following damage due to ischemia, likely due to the subsequent death of the satellite cells and endomysial cells. For this reason, extensive regions of fibrosis are often a dominant feature.
Berridge BR, Van Vleet JF, Herman E. 2013. Cardiac, vascular, and skeletal muscle systems. In: Haschek and Rousseaux’s Handbook of Toxicologic Pathology, 3rd ed (Haschek WM, Rousseaux CG, Wallig MA, Bolon B, Ochoa R, Mahler MW, eds). Elsevier, Amsterdam, 1635-1665.
Greaves P, Chouinard L, Ernst H, Mecklenburg L, Pruimboom-Brees IM, Rinke M, Rittinghausen S, Thibault S, von Erichsen J, Yoshida T. 2013. Proliferative and non-proliferative lesions of the rat and mouse soft tissue, skeletal muscle, and mesothelium. J Toxicol Pathol 26(3 suppl):1S-26S.
Abstract: http://www.ncbi.nlm.nih.gov/pubmed/25035576Haschek WM, Rousseaux CG, Wallig MA. 2010. Cardiovascular and skeletal muscle systems. In: Fundamentals of Toxicologic Pathology, 2nd ed. Academic Press, San Diego, 319-376.
Leininger JR. 1999. Skeletal muscle. In: Pathology of the Mouse (Maronpot R, Boorman G, Gaul BW, eds). Cache River Press, St Louis, 637-643.
Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Muñoz-Cánoves P. 2001. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle 1:21.
Abstract: http://www.ncbi.nlm.nih.gov/pubmed/21798099McDonald MM, Hamilton BF. 1990. Bones, joints, and synovia. In: Pathology of the Fischer Rat: Reference and Atlas (Boorman G, Eustis SL, Elwell MR, Montgomery CA, MacKenzie WF, eds). Academic Press, San Diego, 193-207.
Vahle JL, Leininger JR, Long PH, Hall DG, Ernst H. 2013. Bone, muscle, and tooth. In: Toxicologic Pathology: Nonclinical Safety Assessment (Sahota PS, Popp JA, Hardisty JF, Gopinath C, eds). CRC Press, Boca Raton, FL, 561-587.
Van Vleet JF, Valentine BA. 2007. Muscle and tendon. In: Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, 5th ed, Vol 1 (Grant MG, ed). Elsevier, Edinburgh, 185-280.
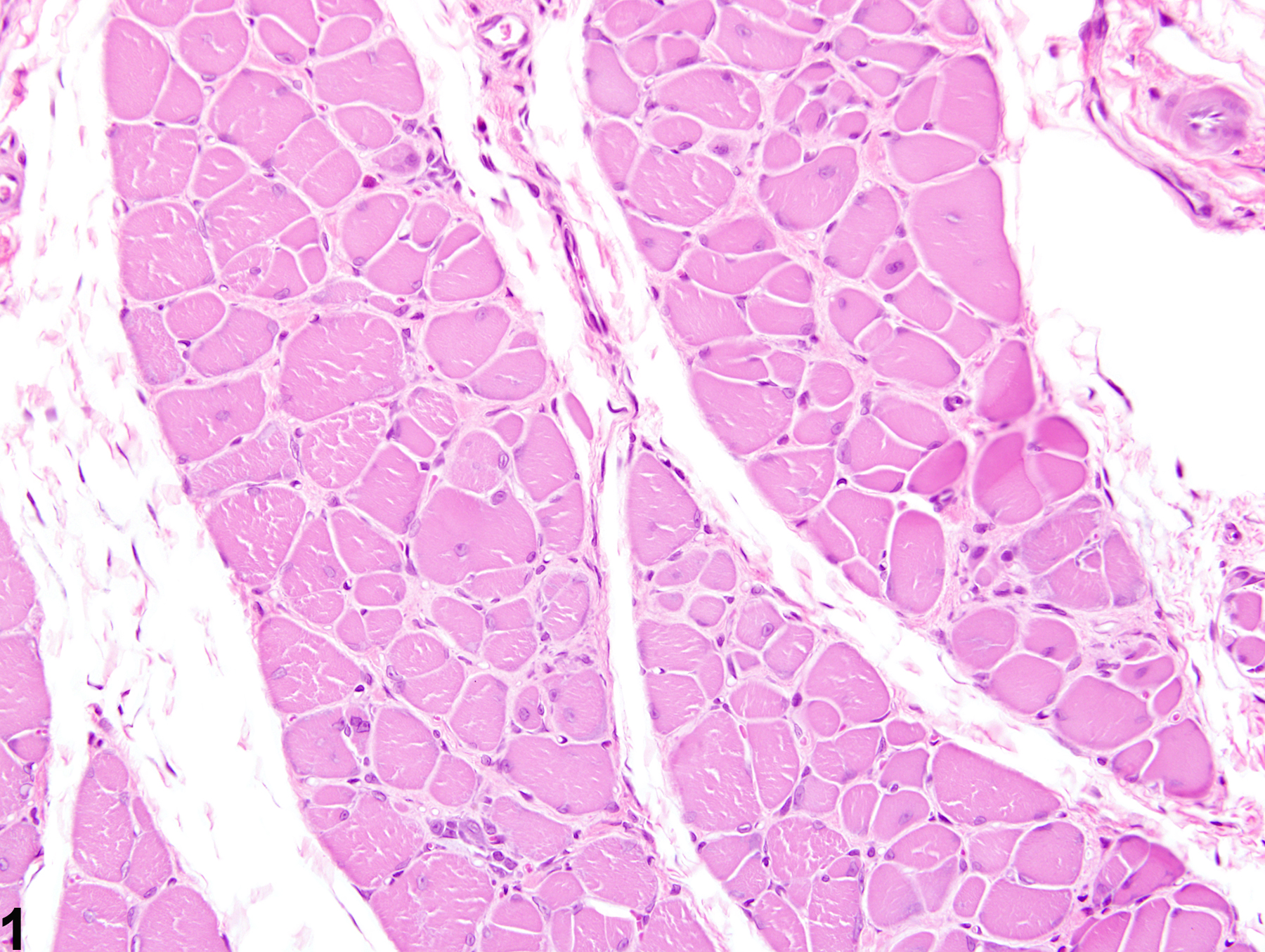
Skeletal muscle - Fibrosis in a male Harlan Sprague-Dawley rat from a subchronic study. Early change consists of increased perimysial deposits of pale eosinophilic material (immature collagen).





