Endocrine System
Adrenal Gland - Hyperplasia
Narrative
Hyperplastic foci in the cortex and medulla are generally rounded, circumscribed areas of variable diameter, characterized by increased numbers of cortical cells. In the cortex, the normal radial cord-like architecture is usually maintained. The hyperplastic foci can be well-demarcated or can blend almost imperceptibly with the adjacent normal tissue; compression is generally absent to minimal. Compared with normal cortical cells, hyperplastic cells are usually but not always smaller, with cytoplasm that can be vacuolated and/or tinctorially different (often more basophilic). In the medulla, foci of hyperplasia are often located at the periphery of the adrenal medulla, at or near the corticomedullary junction (Figure 7 and Figure 8), though they can also occur in the more central medulla (Figure 9 and Figure 10). Compared with normal medullary cells, hyperplastic cells are usually smaller with more basophilic cytoplasm (Figure 7, Figure 8, Figure 9, and Figure 10). In both the cortex and medulla, rare mitotic figures may be present, but the hyperplastic cells typically lack features of cellular atypia.
Cortical and medullary hyperplasia can be a spontaneous aging change, which occurs more commonly in rats than mice. Other causes of cortical hyperplasia include stress, and abnormally elevated adrenocorticotropic hormone (ACTH) levels due to any factor that perturbs the hypothalamic-pituitary-adrenal hormonal axis and/or adrenal steroidogenesis. Medullary hyperplasia can also result from experimental genetic modifications, dietary manipulations, and administration of various chemicals. Hyperplasia may also be a regenerative response to cell loss from degeneration, atrophy, or necrosis.
Focal cortical and medullary hyperplasias are considered proliferative lesions in a morphologic continuum that can progress to neoplasia (adenoma and carcinoma in the cortex and benign and malignant pheochromocytoma in the medulla). Differentiating large focal hyperplasias from smaller adenomas or pheochromocytomas can be challenging, but the neoplasms, compared with hyperplastic foci, are more compressive, exhibit more disorganization, and have some degree of cellular pleomorphism.
Cortical hyperplasia (increased cell numbers) and cortical hypertrophy (increased cell size) can often be concurrent lesions in the same gland or even in the same focus. Thus, as a practical matter, determining whether hyperplasia or hypertrophy is predominant in a given lesion and/or adrenal gland can be very difficult.
Bosland MC, Bär A. 1984. Some functional characteristics of adrenal medullary tumors in aged male Wistar rats. Vet Pathol 21:129-140.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/6730197Brix AE, Nyska A, Haseman JK, Sells DM, Jokinen MO, Walker NJ. 2005. Incidences of selected lesions in control female Harlan Sprague-Dawley rats from two-year studies performed by the National Toxicology Program. Toxicol Pathol 33:477-483.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/16036865Ferreira JC, Cruz CD, Neves D, Pignatelli D. 2007. Increased extracellular signal regulated kinases phosphorylation in the adrenal gland in response to chronic ACTH treatment. J Endocrinol 192:647-658.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/17332532Frith CH. 1983. Pheochromocytoma, adrenal medulla, mouse. In: Monographs on the Pathology of Laboratory Animals: Endocrine System (Jones TC, Mohr U, Hunt RD, eds). Springer, Berlin, 27-30.
Hamlin MH, Banas DA. 1990. Adrenal gland. In: Pathology of the Fischer Rat: Reference and Atlas (Boorman GA, Eustis SL, Elwell MR, Montgomery CA, MacKenzie WF, eds). Academic Press, San Diego, 501-518.
Abstract: https://www.ncbi.nlm.nih.gov/nlmcatalog/9002563McMartin DL, Sahota PS, Gunson DE, Han Hsu H, Spaet RH. 1992. Neoplasms and related proliferative lesions in control Sprague-Dawley rats from carcinogenicity studies. Historical data and diagnostic considerations. Toxicol Pathol 20:212-225.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/1475582National Toxicology Program. 1999. NTP TR-488. Toxicology and Carcinogenesis Studies of 60-Hz Magnetic Fields in F344/N Rats and B6C3F1 Mice (Whole-Body Exposure Studies). NTP, Research Triangle Park, NC.
Abstract: https://ntp.niehs.nih.gov/go/10166National Toxicology Program. 2010. NTP TR-559. Toxicology and Carcinogenesis Studies of 2,3',4,4',5-Pentachlorobiphenyl (PCB 118) (CAS No. 31508-00-6) in Female Harlan Sprague-Dawley Rats (Gavage Studies). NTP, Research Triangle Park, NC.
Abstract: https://ntp.niehs.nih.gov/go/33539National Toxicology Program. 2012. NTP TR-573 Toxicology and Carcinogenesis Studies of Styrene-Acrylonitrile Trimer in F344/N Rats (Perinatal and Postnatal Feed Studies). NTP, Research Triangle Park, NC.
Abstract: https://ntp.niehs.nih.gov/go/36150National Toxicology Program. 2012. NTP TR-576. Toxicology and Carcinogenesis Studies of Trimethylpropane Triacrylate (Technical Grade) (CAS No. 15625-89-5) in F344/N Rats and B6C3F1/N Mice (Dermal Studies). NTP, Research Triangle Park, NC.
Abstract: https://ntp.niehs.nih.gov/go/37176National Toxicology Program. 2013. NTP TR-578. Toxicology and Carcinogenesis Studies of Gingko biloba Extract in F344/N Rats and B6C3F1 Mice (Gavage Studies). NTP, Research Triangle Park, NC.
Abstract: https://ntp.niehs.nih.gov/go/37193Nyska A, Maronpot RR. 1990. Adrenal gland. In: Pathology of the Mouse: Reference and Atlas (Maronpot RR, Boorman GA, Gaul BW, eds). Cache River Press, Vienna, IL, 509-536.
Abstract: http://www.cacheriverpress.com/books/pathmouse.htmPanagiotidou E, Zerva S, Mitsiou DJ, Alexis MN, Kitraki E. 2014. Perinatal exposure to low-dose bisphenol A affects the neuroendocrine stress response in rats. J Endocrinol 220:207-218.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/24323913Rosol TJ, Yarrington JT, Latendresse J, Capen CC. 2001. Adrenal gland: Structure, function, and mechanisms of toxicity. Toxicol Pathol 29:41-48.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/11215683Stout MD, Herbert RA, Kissling GE, Suarez F, Roycroft JH, Chhabra RS, Bucher JR. 2008. Toxicity and carcinogenicity of methyl isobutyl ketone in F344N rats and B6C3F1 mice following two year inhalation exposure. Toxicology 244:209-219.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/18178301Strandberg JD. 1983. Focal hyperplasia, adrenal cortex, rat. In: Monographs on the Pathology of Laboratory Animals: Endocrine System (Jones TC, Mohr U, Hunt RD, eds). Springer, Berlin, 37-41.
Abstract: http://www.springer.com/medicine/pathology/book/978-3-642-64649-2Taylor I. 2011. Mouse. In: Background Lesions in Laboratory Animals: A Color Atlas (McInnes EF, ed). Saunders Elsevier, Amsterdam, 45-72.
Abstract: http://www.sciencedirect.com/science/book/9780702035197Tischler AS, Powers JF, Alroy J. 2004. Animal models of pheochromocytoma. Histol Histopathol 19:883-895.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/15168351Tischler AS, Powers JF, Downing JC, Risberg JC, Shahsavri M, Ziar J, McClain RM. 1996. Vitamin D3, lactose, and xylitol stimulate chromaffin cell proliferation in the rat adrenal medulla. Toxicol Appl Pharmacol 140:115-123.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/8806877Tischler AS, Sheldon W. 1996. Adrenal medulla. In: Pathobiology of the Aging Mouse, Vol 1 (Mohr U, Dungworth DL, Capen CC, Carlton WW, Sundberg JP, JM Ward, eds). International Life Sciences Institute, Washington, DC, 135-151.
Tonks ID, Mould AW, Schroder WA, Cotterill A, Hayward NK, Walker GJ, Kay GF. 2010. Dual loss of Rb1 and Trp53 in the adrenal medulla leads to spontaneous pheochromocytoma. Neoplasia 12:235-243.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/20234817Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. 2006. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol (Endocrinol Metab) 291:E965-E973.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/16772325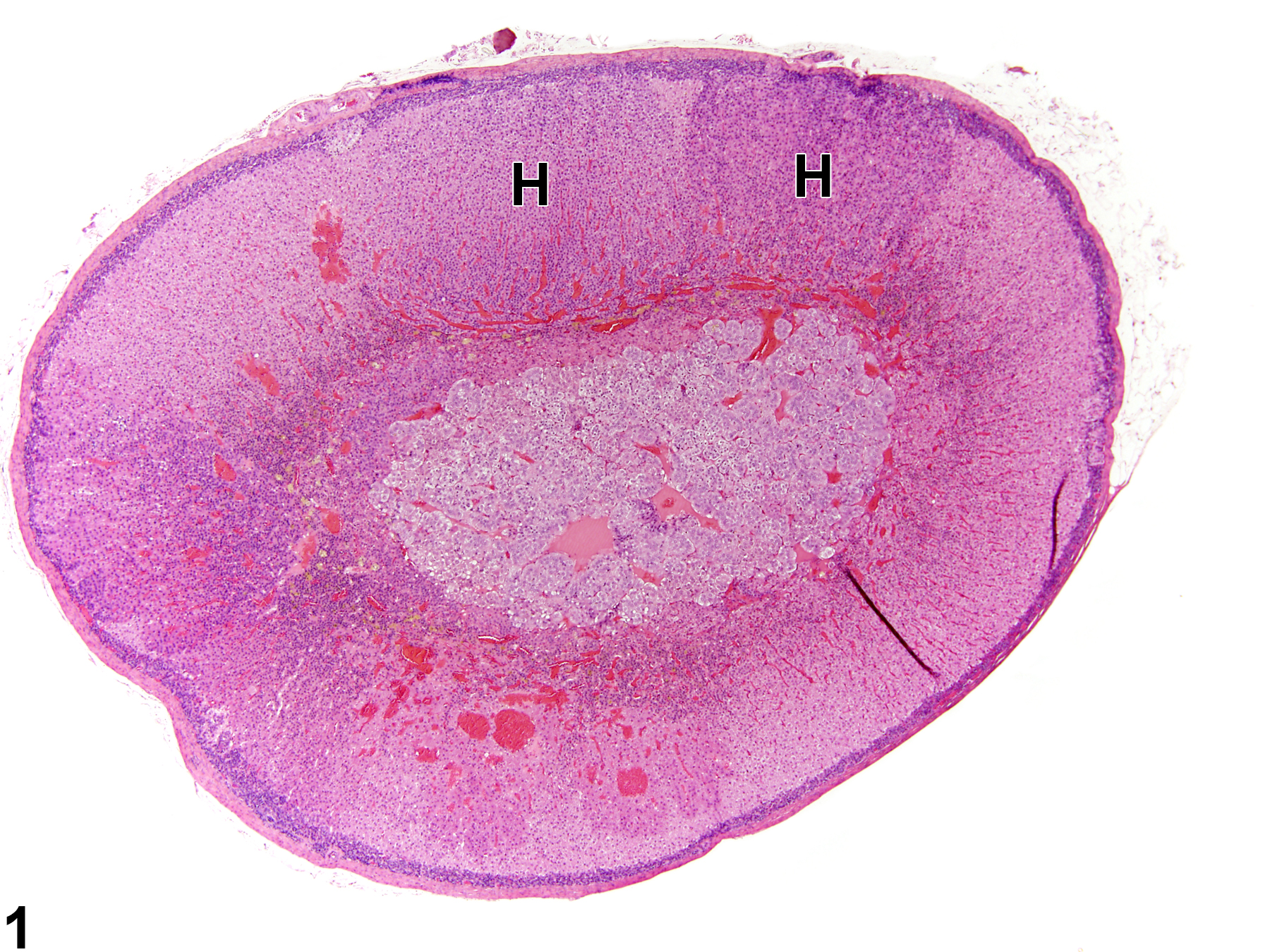
Adrenal gland, Cortex - Hyperplasia in a male Sprague-Dawley rat from a chronic study. There are two adjacent foci of hyperplasia (H) in the zona fasciculata.











