Special Senses System
Harderian Gland - Inflammation
Narrative
In NTP studies, there are five standard categories of inflammation: acute, suppurative, chronic, chronic-active, and granulomatous. In acute inflammation, the predominant infiltrating cell is the neutrophil, though fewer macrophages and lymphocytes may also be present. There may also be evidence of edema or hyperemia. The neutrophil is also the predominant infiltrating cell type in suppurative inflammation, however, in suppurative inflammation, the neutrophils are aggregated and many of them are degenerate (suppurative exudate). Cell debris, both from the resident cell populations and infiltrating leukocytes, proteinaceous fluid containing fibrin, fewer macrophages, occasional lymphocytes or plasma cells, and, possibly, an infectious agent may also be present in within the exudate. Grossly, these lesions would be characterized by the presence of pus. In the tissue surrounding the exudate, there may be fibroblasts, fibrous connective tissue, and mixed inflammatory cells, depending on the chronicity of the lesion. Lymphocytes predominate in chronic inflammation. Lymphocytes also predominate in chronic-active inflammation, but in chronic-active inflammation, there are also a significant number of neutrophils. Both lesions may contain macrophages. Granulomatous inflammation is another form of chronic inflammation, but this diagnosis requires the presence of a significant number of aggregated, large, activated macrophages, epithelioid macrophages, or multinucleated giant cells. Inflammation differs from cellular infiltration in that inflammatory lesions have evidence of tissue damage, such as edema, hemorrhage, degeneration, necrosis, regeneration, etc.
Beaumont SD. 2002. Ocular disorders of pet mice and rats. Vet Clin Exot Anim 5:311-324.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/12170635Botts S, Jokinen M, Gaillard ET, Elwell MR, Mann PC. 1999. Salivary, Harderian, and lacrimal glands. In: Pathology of the Mouse: Reference and Atlas (Maronpot RR, Boorman GA, Gaul BW, eds). Cache River Press, Vienna, IL, 49-79.
Abstract: http://www.cacheriverpress.com/books/pathmouse.htmGreaves P. 2007. Nervous system and special sense organs. In: Histopathology of Preclinical Toxicity Studies: Interpretation and Relevance in Drug Safety Evaluation, 3rd ed. Academic Press, San Diego, CA, 861-933.
Abstract: http://www.sciencedirect.com/science/book/9780444527714Krinke AL, Schaetti PR, Krinke GJ. 1994. Changes in the major ocular glands. In: Pathobiology of the Aging Rat, Vol 1 (Mohr U, Dungworth DL, Capen CC, Carlton WW, Sundberg JP, Ward JM, eds). International Life Sciences Institute Press, Washington, DC, 109-119.
Krinke GJ, Schaetti PR, Krinke A. 1996. Nonneoplastic and neoplastic changes in the Harderian and lacrimal glands. In: Pathobiology of the Aging Mouse, Vol 2 (Mohr U, Dungworth DL, Capen CC, Carlton WW, Sundberg JP, Ward JM, eds). International Life Sciences Institute Press, Washington, DC, 139-152.
Lambert RA, Yudkin AM. 1923. Changes in the paraocular glands accompanying the ocular lesions which result from a deficiency of vitamine A. J Exp Med 38:25-32.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/19868768National Toxicology Program. 1989. NTP TR-345. Toxicology and Carcinogenesis Studies of Roxarsone (CAS No. 121-19-7) in F344/N Rats and B6C3F1 Mice (Feed Studies). NTP, Research Triangle Park, NC.
Abstract: https://ntp.niehs.nih.gov/go/8644National Toxicology Program. 1989. NTP TR-350. Toxicology and Carcinogenesis Studies of Tribromomethane (Bromoform) (CAS No. 75-25-2) in F344/N Rats and B6C3F1 Mice (Gavage Studies). NTP, Research Triangle Park, NC.
Abstract: https://ntp.niehs.nih.gov/go/6961National Toxicology Program. 2007. NTP TR-526. Toxicology and Carcinogenesis Studies of a Mixture of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) (CAS No. 1746-01-6), 2,3,4,7,8-Pentachlorodibenzofuran (PeCDF) (CAS No. 57117-31-4), and 3,3',4,4',5-Pentachlorobiphenyl (PCB 126) (CAS No. 57465-28-8) in Female Harlan Sprague-Dawley Rats (Gavage Studies). NTP, Research Triangle Park, NC.
Abstract: https://ntp.niehs.nih.gov/go/9307O’Steen WK, Kraeer SL, Shear CR. 1978. Extraocular muscle and Harderian gland degeneration and regeneration after exposure of rats to continuous fluorescent illumination. Invest Ophthalmol Vis Sci 17:847-856.
Abstract: https://pubmed.ncbi.nlm.nih.gov/700964/Payne AP. 1994. The Harderian gland: A tercentennial review. J Anat 185:1-49.
Abstract: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1166813/Percy DH, Wojcinski ZW, Schunk MK. 1989. Sequential changes in the Harderian and exorbital lacrimal glands in Wistar rats infected with sialodacryoadenitis virus. Vet Pathol 26:238–245.
Full Text: http://vet.sagepub.com/content/26/3/238.full.pdfRothwell TLW , Everitt AV. 1986. Exophthalmos in ageing rats with Harderian gland disease. Lab Anim 20:97-100.
Abstract: http://lan.sagepub.com/content/20/2/97.short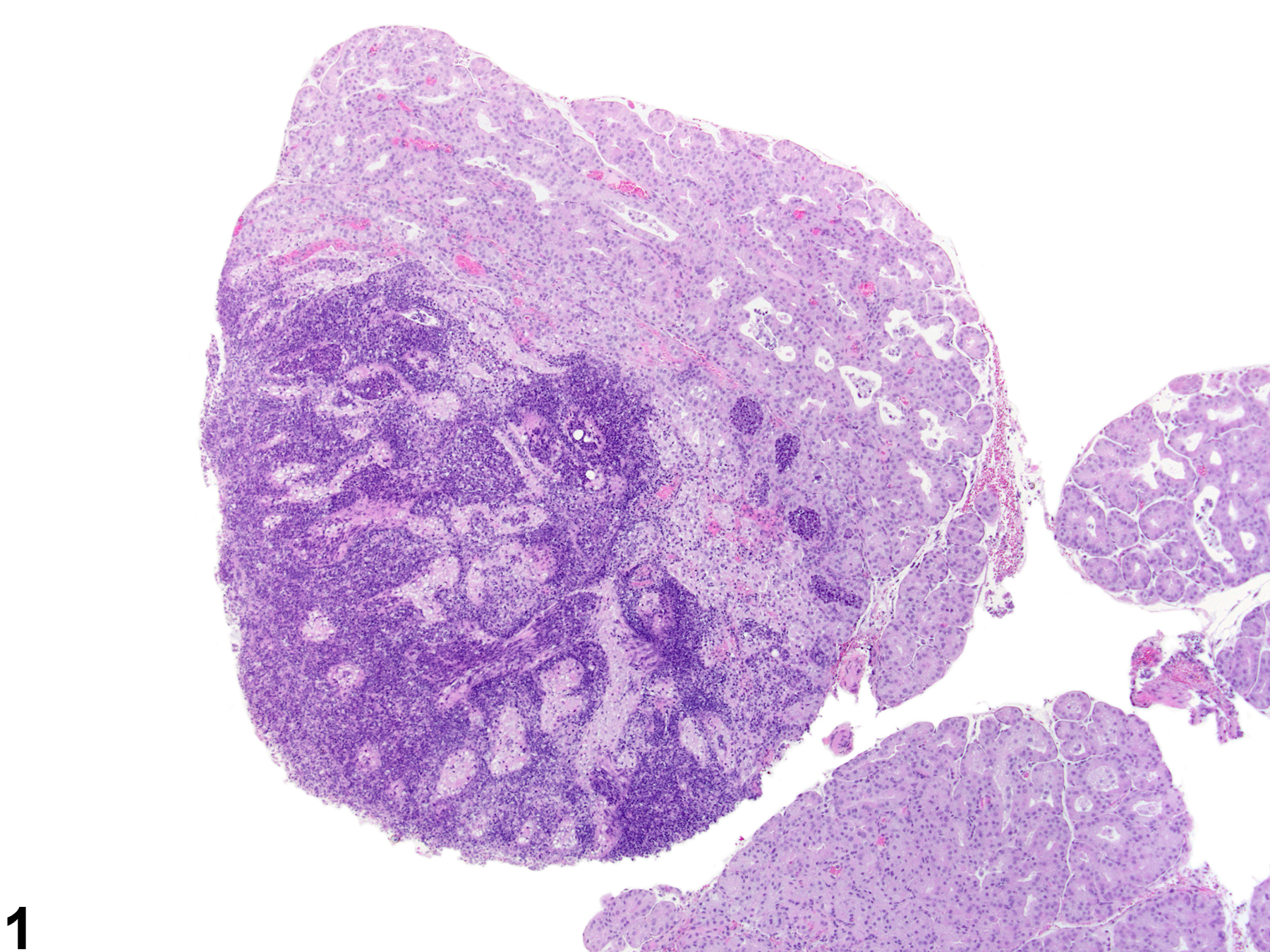
Harderian gland - Inflammation, Chronic active in a female Sprague- Dawley rat from a chronic study. Numerous inflammatory cells are present in the interstitium and alveoli with destruction of the acinar tissue.





