Nervous System
Brain - Mineralization
Narrative
On the other hand, mineral deposits, secondary to necrosis, are attributed to the commonly observed but complex process of dystrophic mineralization. Dystrophic mineralization is often seen as lamellar calcospherites found secondary to small clustered regions of neuronal necrosis at various neural sites, such as depicted in Figure 3 and Figure 4. Figure 3 shows the aggregation of mineralized bodies (arrow) in sites of former granule cell necrosis in the cerebellum. Note the loss of neural tissue represented by spaces formerly occupied by internal granule cells, and the presence of multiple small, dark, round bodies. The presence of such mineralized foci may lead to disruption of the tissue during microtomy. The mineralized foci shown in Figure 3 should not be confused with the artifactual presence of boney spicules often carried into brain from overlying skull fragments during brain extraction/trimming or with heterotopic bone tissue. The round, often concentric, lamellar appearance of calcospherites, shown at higher magnification in Figure 4, is typical of late stages of mineralization of necrotic brain tissue. Calcospherites are deposited in a region of former cerebellar internal granule cell necrosis. Note the concentric laminated structures (arrow) of the larger bodies that are apparently absent in smaller ones. The deposition of mineral in the form of calcospherites in these cases requires several weeks to form after the necrotizing insult.
Figure 5 shows a region of prior necrosis adjacent to piriform cortex with an uncommon reactive focus of osteoid (arrow) and ossification (arrowhead). A large zone of brain necrosis is replaced by a progression of mineralized bodies of several types. To the right are some smaller basophilic calcospherites, and to the left are larger zones of necrotic brain tissue transformed into both osteoid, composed of pink hyaline material, and a more advanced stage of osseous metaplasia, where dense basophilic mineral and osteocytes are apparent.
Eidelman N, Boyde A, Bushby AJ, Howell PGT, Sun J, Newbury DE, Miller FW, Robey PG, Rider LG. 2009. Microstructure and mineral composition of dystrophic calcification associated with the idiopathic inflammatory myopathies. Arthritis Res Ther 11(5):R159.
Abstract: http://www.ncbi.nlm.nih.gov/pubmed/19857267Kalueff A, Loseva E, Haapasalo H, Tuchimaa P. 2006. Thalamic mineralization in vitamin D receptor knock-out mice. Neuroreport 17:717-721.
Abstract: http://www.ncbi.nlm.nih.gov/pubmed/16641675Landis WJ. 1996. Mineral characterization in calcifying tissues: Atomic, molecular and macromolecular perspectives. Connect Tissue Res 34:239-246.
Abstract: http://www.ncbi.nlm.nih.gov/pubmed/9084632Morgan KT, Johnson BP, Frith CH, Townsend J. 1982. An ultrastructural study of spontaneous mineralization in the brains of ageing mice. Acta Neuropathol 58:120-124.
Abstract: http://www.ncbi.nlm.nih.gov/pubmed/7180386Roach HI. 1992. Induction of normal and dystrophic mineralization by glycerophosphates in long-term bone organ culture. Calcif Tissue Int 50:553-563.
Abstract: http://www.ncbi.nlm.nih.gov/pubmed/1525713Yanai T, Yamoto T, Manabe J, Takaoka M, Matsunuma N. 1987. X-ray microanalysis of mineralization in the thalamus of aged mice. Jap J Vet Sci 49:920-922.
Abstract: http://www.ncbi.nlm.nih.gov/pubmed/3682532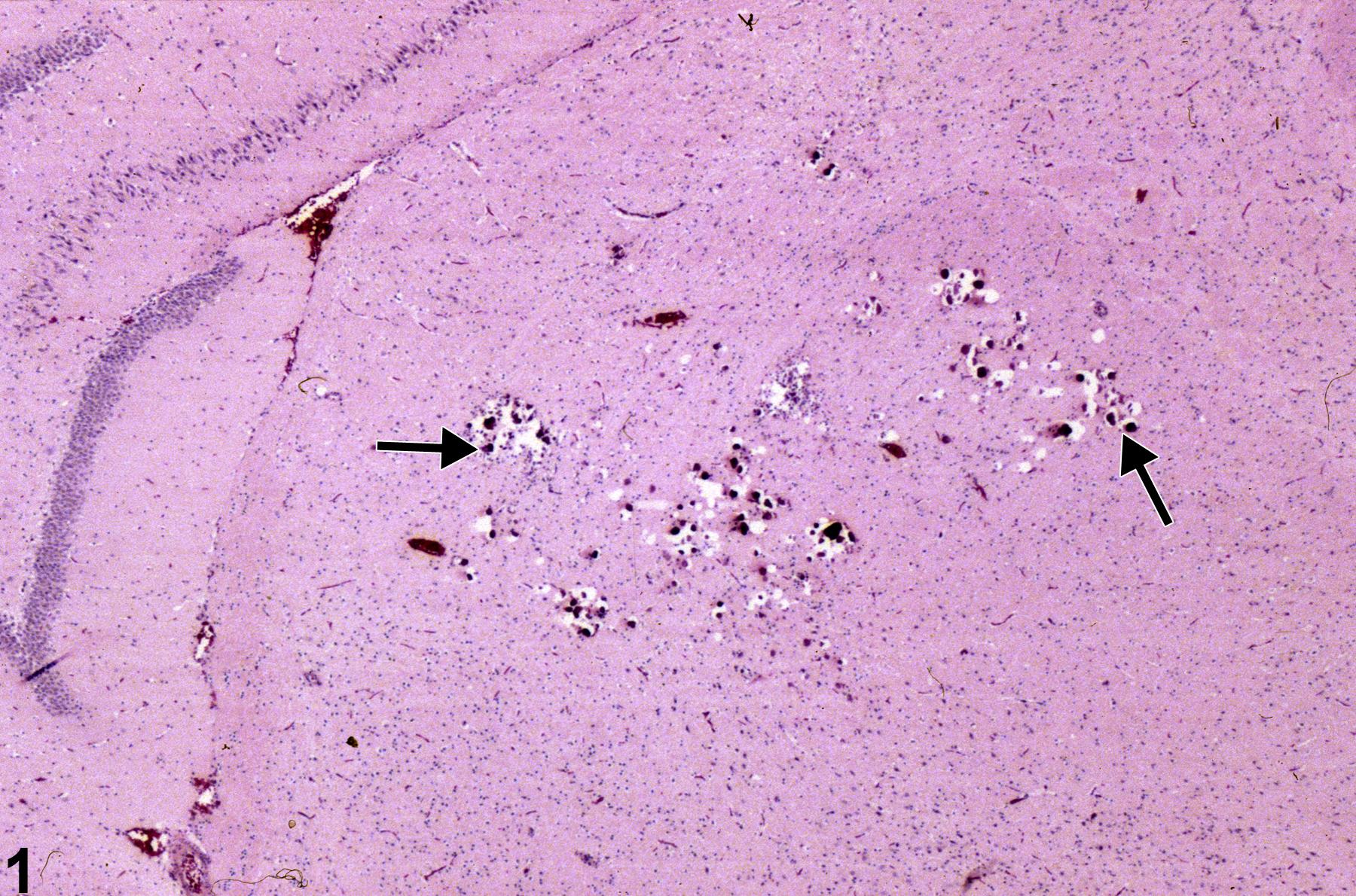
Incidental thalamic mineralization in a female F344/N rat from a 16-week study. The arrows identify focal mineral deposits.






