Respiratory System
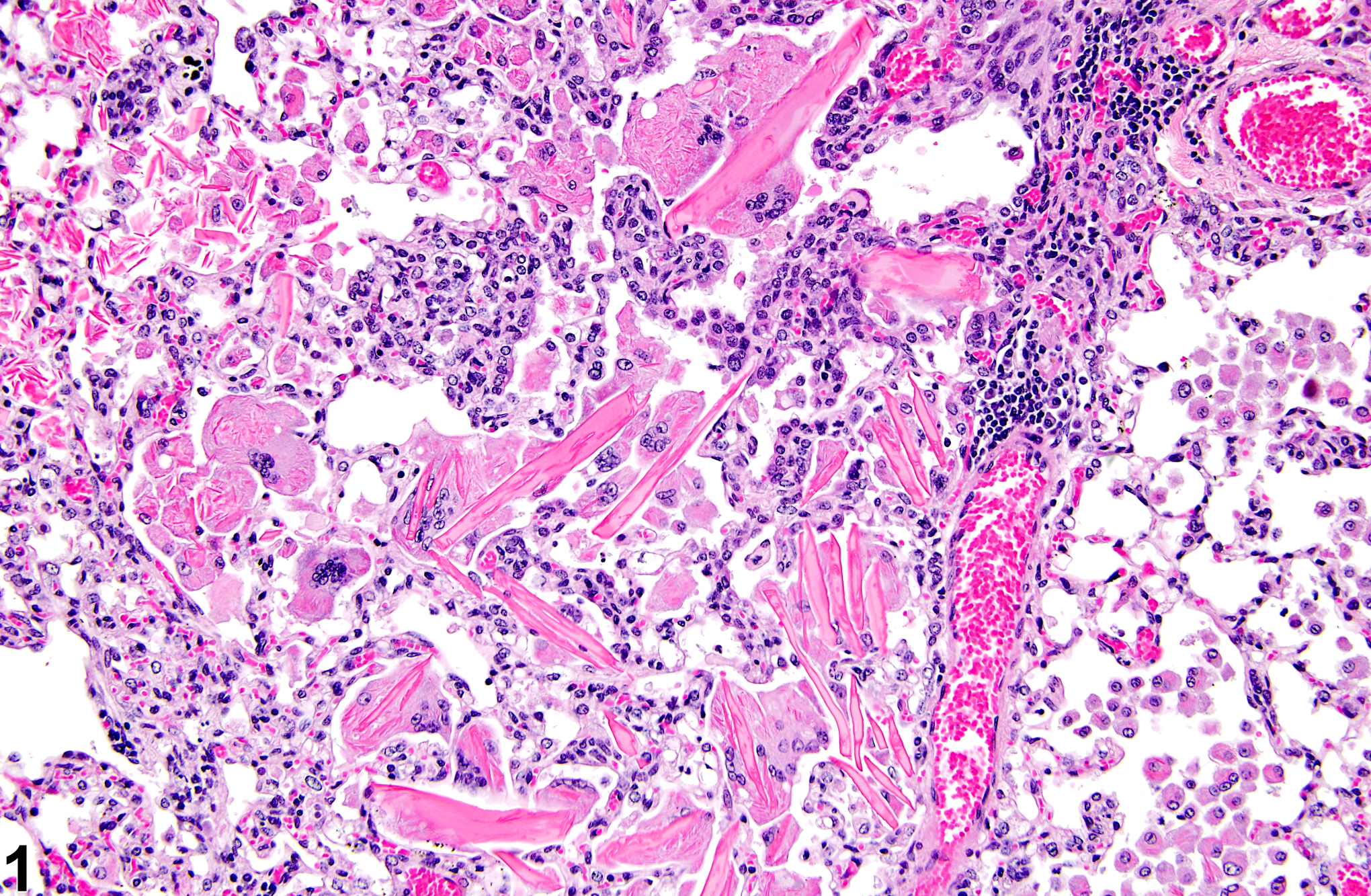
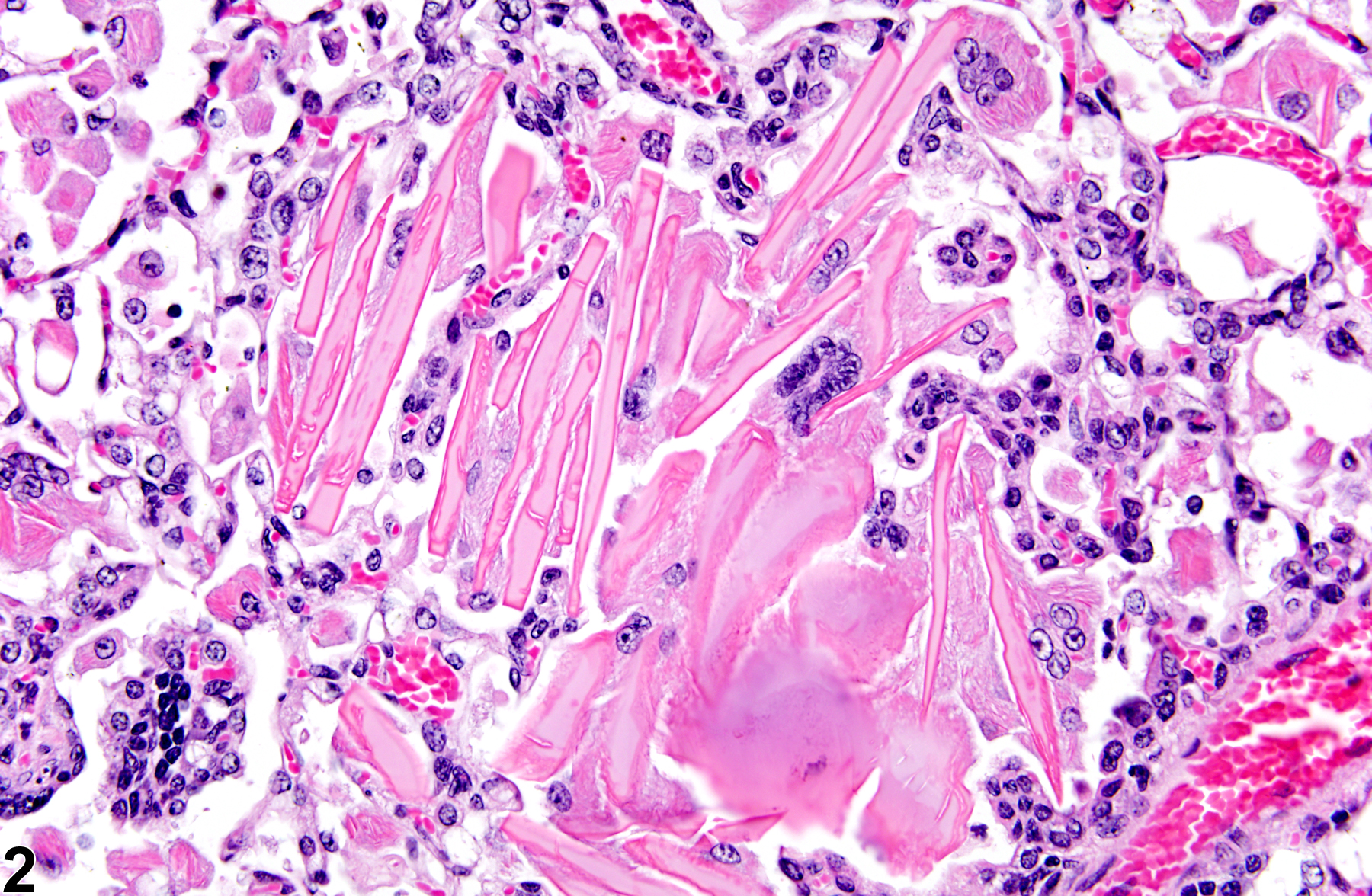
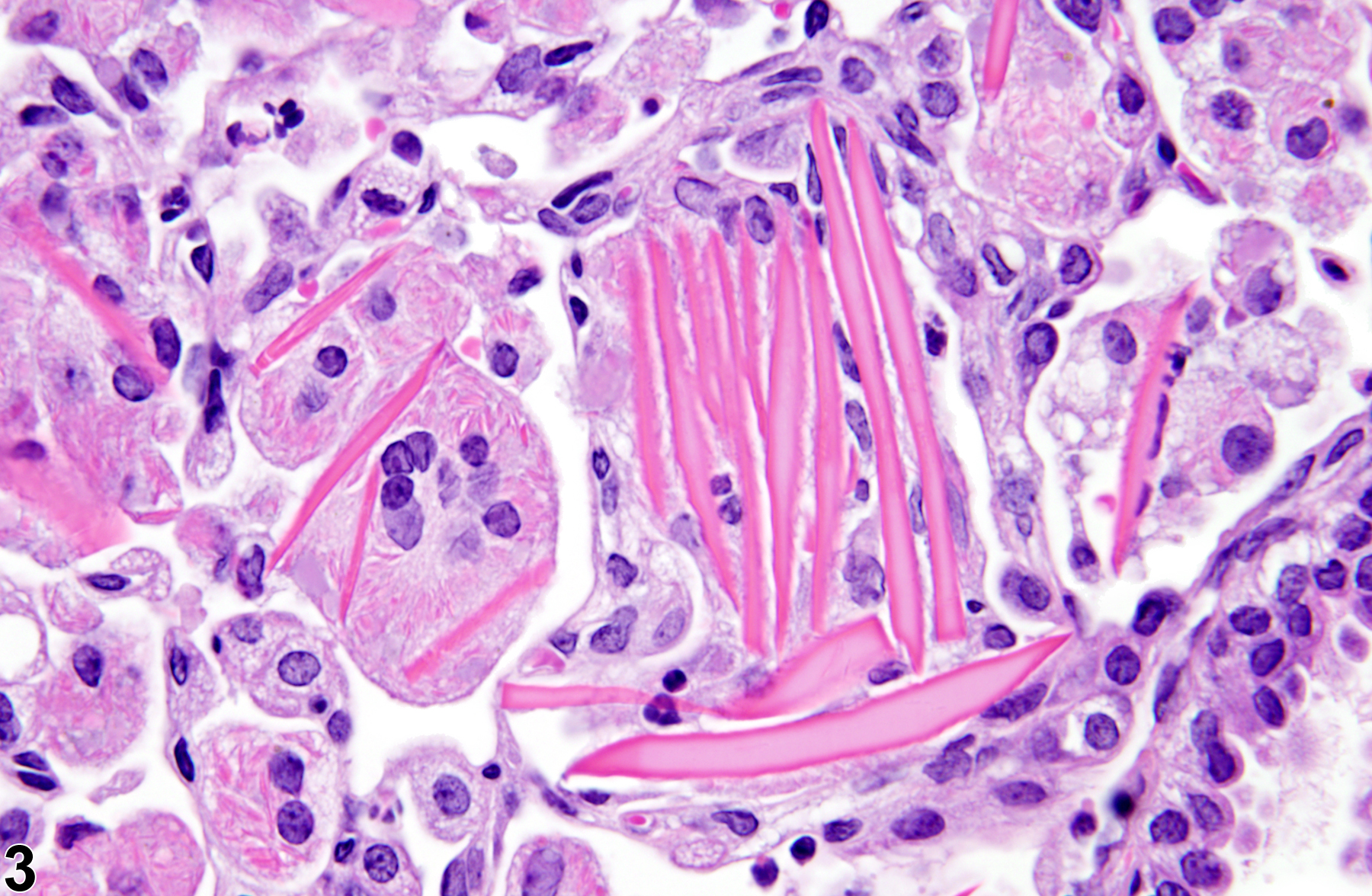
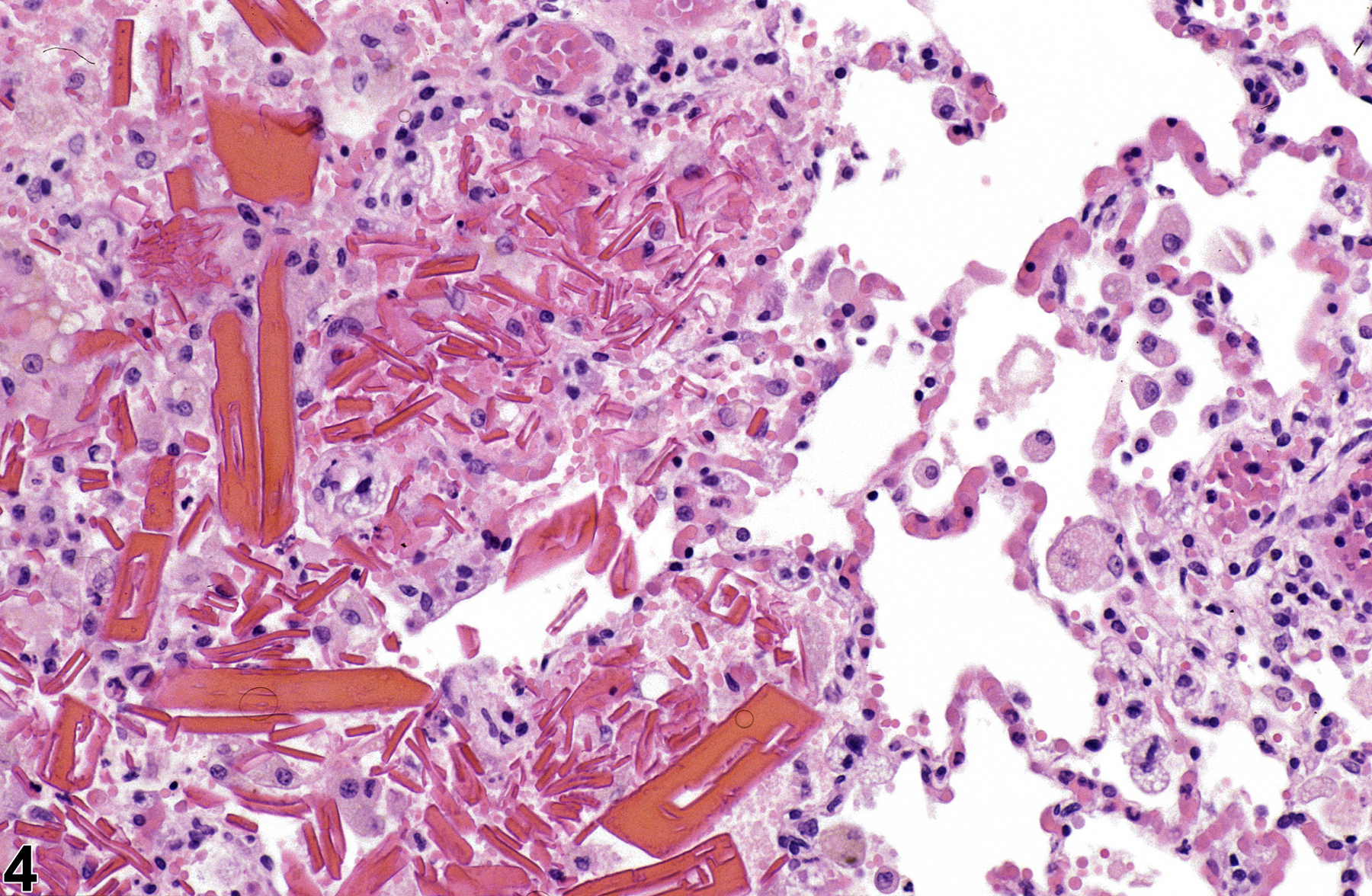
Lung - Crystals
Narrative
Hemoglobin crystals are occasionally seen in areas of hemorrhage (Figure 4), often associated with inflammation. This type of crystal is most commonly seen in rats because rat hemoglobin crystallizes more readily than does hemoglobin in other species. Only structurally abnormal hemoglobin crystallizes in vivo in other species. Hemoglobin crystals are typically rectangular and, as opposed to the eosinophilic crystals of eosinophilic crystalline pneumonia, form in the extracellular space, though smaller hemoglobin crystals may be phagocytosed by alveolar macrophages. They typically resemble red blood cells in color and are usually spatially associated with extravasated erythrocytes.
Eosinophilic crystalline pneumonia is an idiopathic disease that occurs in mice. It can be a sporadic background lesion, with the severity varying from mild and subclinical to severe, in which case it may cause death. It may be seen in some genetically engineered mice (e.g., p47phox-/- mice) or mouse strains developed from naturally occurring mutations (e.g., Ptpn6me motheaten mice). Susceptible strains include C57BL/6 and 129Sv mice. The pathogenesis of eosinophilic crystalline pneumonia is unclear. It has been associated with eosinophilic inflammation in susceptible strains, such as that caused by parasitic and fungal infections, though the main cell type associated with the crystals is the alveolar macrophage. The crystals are immunoreactive to anti-Ym1 protein antibodies (a.k.a. T-lymphocyte-derived eosinophil chemotactic factor). Eosinophilic crystalline pneumonia is histologically similar to the crystals (and inflammation) associated with inhalation of particulates but does not occur with any relation to treatment and would also be seen in control mice.
Other types of crystals have been identified in rodent lungs. Subchronic exposure of rats to clofazimine, an antileprotic drug, leads to deposition of reddish orange crystals in many tissues, including lung. Chronic dietary administration of kojic acid in F344 rats produced pulmonary microgranulomas containing crystals. Cryptococcus neoformans infection in C57BL/6 mice generated rod-like crystalline structures found associated with yeast cells or free in host cell cytoplasm. Though these crystals appear morphologically similar to those seen in murine eosinophilic crystalline pneumonia, these crystals are presumed to be the product of interaction of a host protein and a capsular cryptococcal polysaccharide.
Feldmesser M, Kress Y, Casadevall A. 2001. Intracellular crystal formation as a mechanism of cytotoxicity in murine pulmonary Cryptococcus neoformans infection. Infect Immun 69:2723-2727.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/11254641Guo L, Johnson RS, Schuh JC. 2000. Biochemical characterization of endogenously formed eosinophilic crystals in the lungs of mice. J Biol Chem 275:8032-8037.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/10713123Hoenerhoff MJ, Starost MF, Ward JM. 2006. Eosinophilic crystalline pneumonia as a major cause of death in 129S4/SvJae mice. Vet Pathol 43:682-688.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/16966445Kleinig TJ, Helps SC, Ghabriel MN, Manavis J, Leigh C, Blumbergs PC, Vink R. 2009. Hemoglobin crystals: A pro-inflammatory potential confounder of rat experimental intracerebral hemorrhage. Brain Res 1287:164-172.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/19576188Liu Q, Cheng LI, Yi L, Zhu N, Wood A, Changpriroa CM, Ward JM, Jackson SH. 2009. p47phox deficiency induces macrophage dysfunction resulting in progressive crystalline macrophage pneumonia. Am J Pathol 174:153-163.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/19095958Mamidi NV, Rajasekhar A, Prabhakar MC, Krishna DR. 1995. Tissue distribution and deposition of clofazimine in rat following subchronic treatment with or without rifampicin. Arzneimittelforschung 45:1029-1031.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/7488306Murray AB, Luz A. 1990. Acidophilic macrophage pneumonia in laboratory mice. Vet Pathol 27:274-281.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/2169666Ota Y, Imai T, Onose J, Takami S, Cho YM, Hirose M, Nishikawa A. 2009. A 55-week chronic toxicity study of dietary administered kojic acid (KA) in male F344 rats. J Toxicol Sci 34:305-313.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/19483384Paakko P, Anttila S, Sormunen R, Ala-Kokko L, Peura R, Ferrans VJ, Ryhanen L. 1996. Biochemical and morphological characterization of carbon tetrachloride-induced lung fibrosis in rats. Arch Toxicol 70:540-552.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/8831904Ward JM. 1978. Pulmonary pathology of the motheaten mouse. Vet Pathol 15:170-178.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/664185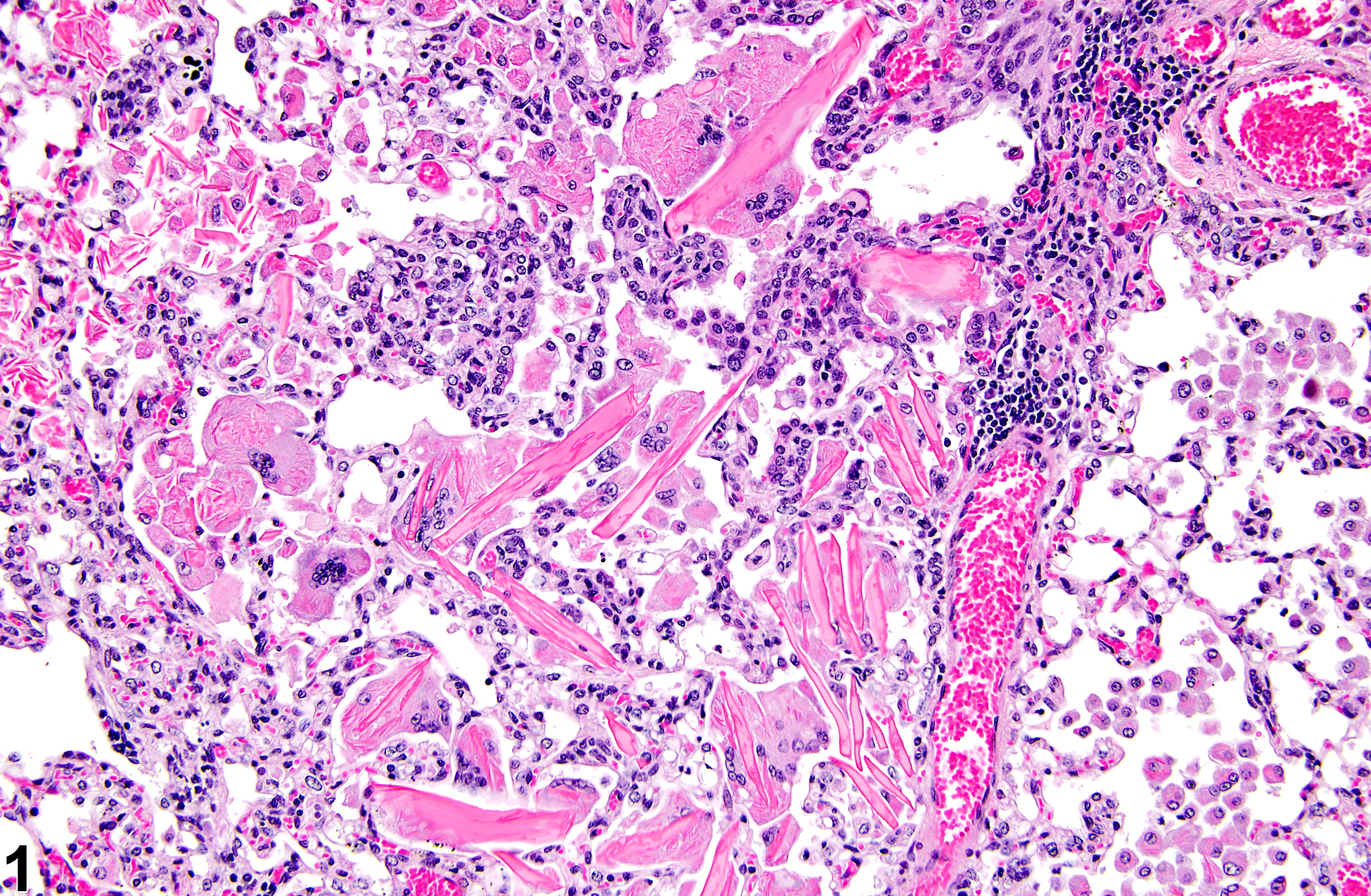
Lung - Crystals in a male B6C3F1/N mouse from a chronic study. Acicular, eosinophilic crystals and associated granulomatous inflammation are present in the alveoli.