Musculoskeletal System
Bone - Fibro-osseous Lesion
Narrative
Fibro-osseous lesions (FOLs) arise commonly within the sternebrae, vertebrae, tibias, femurs, and other bones in a variety of mouse strains. The incidence of FOL is higher in B6C3F1 mice than in other strains, and it is the most common primary bone lesion in B6C3F1 mice. This lesion has not been reported in the rat.
These lesions are characterized by partial or complete replacement of bony trabeculae and marrow cavity by fibrovascular stroma containing fibroblasts, osteoclasts, and osteoblasts embedded in eosinophilic matrix (Figure 1 and Figure 2). The histopathologic features are similar to fibrous osteodystrophy; however, FOLs occur in the absence of parathyroid or renal alterations, typically arise as focal lesions within the metaphyseal or endocortical regions (Figure 3), and may progress to involve larger areas of the bone (Figure 4).
FOL increases in incidence with age and arises most often in female mice (40–100% incidence in B6C3F1 females, <1% in males), suggesting involvement of estrogens. These lesions are often accompanied by alterations in the uterus or ovary consistent with hyperestrogenism (endometrial cystic hyperplasia, vaginal epithelial cell hyperplasia and hyperkeratosis, ovarian follicular development/atresia and cysts). However, these lesions also arise in ovariectomized female mice and castrated males.
Historically, FOL has been referred to by a variety of names, including fibro-osseous dysplasia, fibrous dysplasia, focal osteodystrophy, osteodysplasia, osteofibrosis, and osteodystrophy, but the preferred term for these lesions is "fibro-osseous lesion."
Albassam MA, Courtney CL. 1996. Non-neoplastic and neoplastic lesions of the bone. In: Pathobiology of the Aging Mouse, Vol 2 (Mohr U, Dungworth DL, Capen CC, Carlton WW, Sundberg JP, Ward JM, eds). ILSI Press, Washington, DC, 425-437.
Albassam MA, Wojcinski ZW, Barsoum NJ, Smith GS. 1991. Spontaneous fibro-osseous proliferative lesions in the sternums and femurs of B6C3F1 mice. Vet Pathol 28:381-388.
Abstract: http://www.ncbi.nlm.nih.gov/pubmed/1750163Dodd DC, Port CD. 1987. Hyperostosis of the marrow cavity caused by misoprostol in CD-1 strain mice. Vet Pathol 24:545-548.
Abstract: http://www.ncbi.nlm.nih.gov/pubmed/3137716Gervais F, Attia MA. 2005. Fibro-osseous proliferation in the sternums and femurs of female B6C3F1, C57black and CD-1 mice: A comparative study. Dtsch Tierarztl Wochenschr 112:323-326.
Abstract: http://www.ncbi.nlm.nih.gov/pubmed/16240910Greenman DL, Delongchamp RR. 1986. Interactive response to diethylstilbestrol in C3H mice. Food Chem Toxicol 24:931-934.
Abstract: http://www.ncbi.nlm.nih.gov/pubmed/3781439Highman B, Roth SI, Greenman DL. 1981. Osseous changes and osteosarcomas in mice continuously fed diets containing diethylstilbestrol or 17 beta-estradiol. J Natl Cancer Inst 67:653-662.
Abstract: http://www.ncbi.nlm.nih.gov/pubmed/6944535McAnulty PA, Skydsgaard M. 2005. Diethylstilbestrol (DES): Carcinogenic potential in Xpa-/-, Xpa-/- / p53+/-, and wild-type mice during 9 months' dietary exposure. Toxicol Pathol 33:609-620.
Abstract: http://www.ncbi.nlm.nih.gov/pubmed/16178126Sass B, Montali RJ. 1980. Spontaneous fibro-osseous lesions in aging female mice. Lab Anim Sci 30:907-909.
Abstract: http://www.ncbi.nlm.nih.gov/pubmed/7431877Travlos G. 2006. Histopathology of bone marrow. Toxicol Pathol 34:566-598.
Abstract: http://www.ncbi.nlm.nih.gov/pubmed/17067944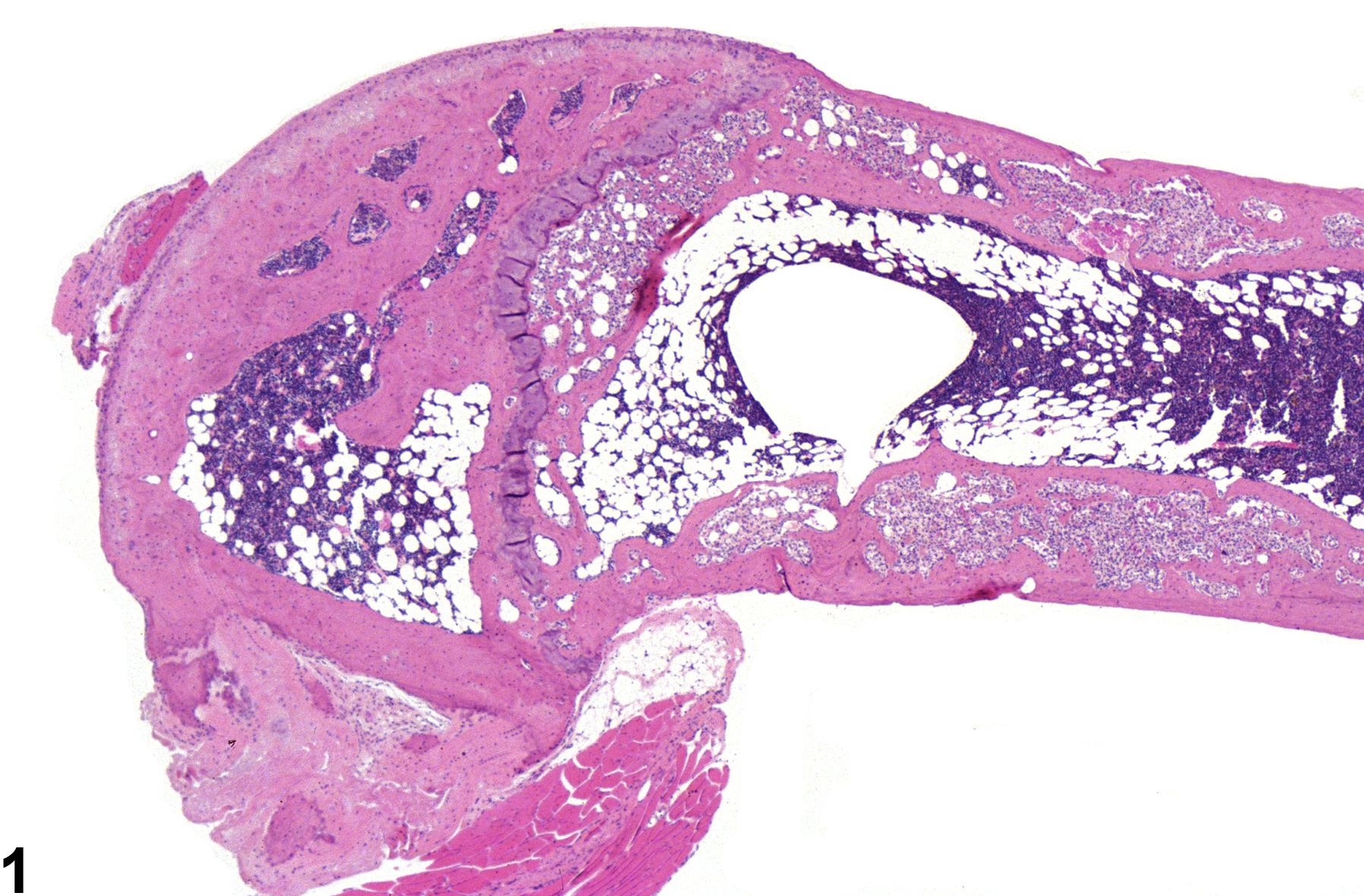
Bone - Fibro-osseous lesion in the femur of a female B6C3F1/N mouse from a chronic study.





