Hepatobiliary System
Liver, Hepatocyte - Hypertrophy
Narrative
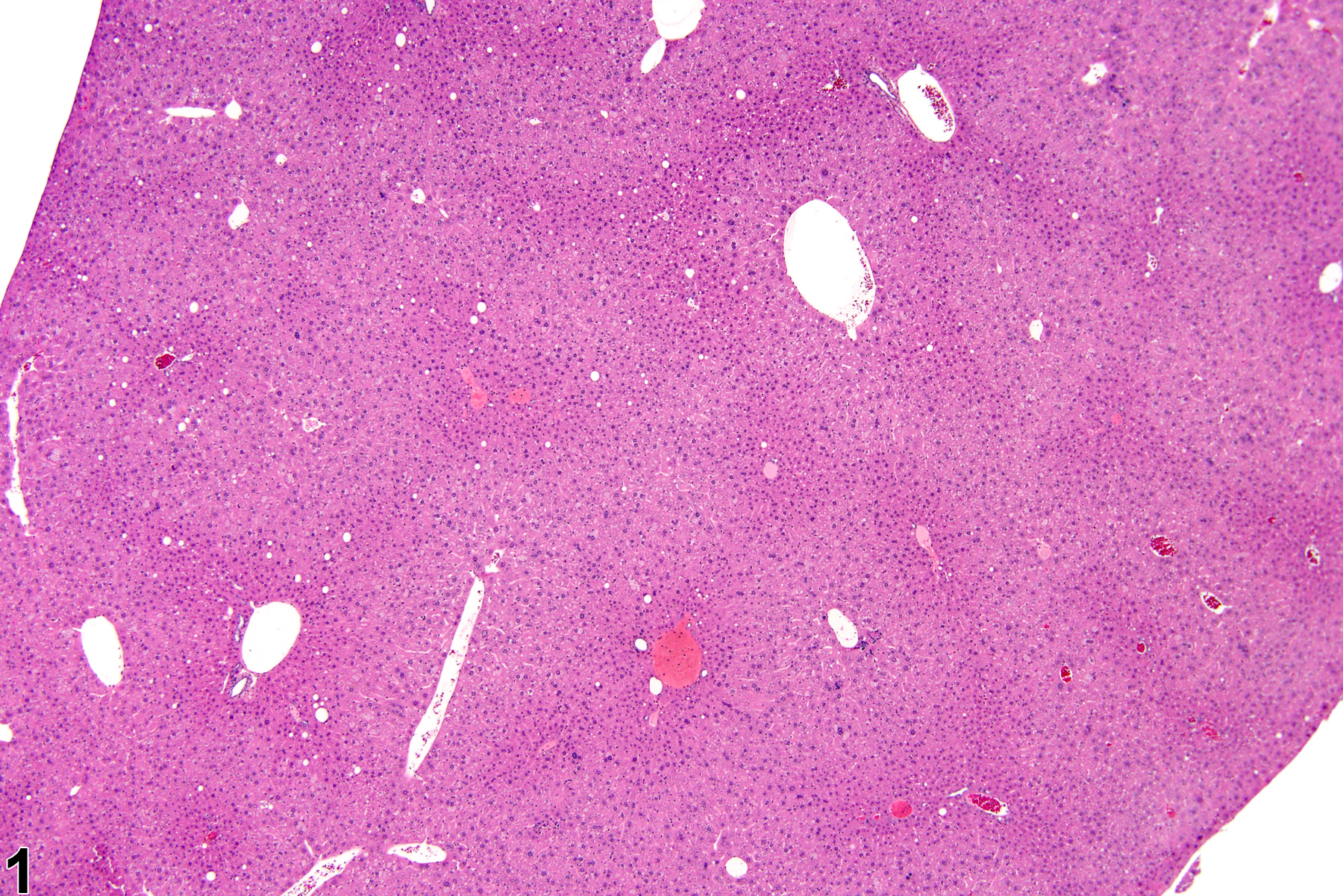
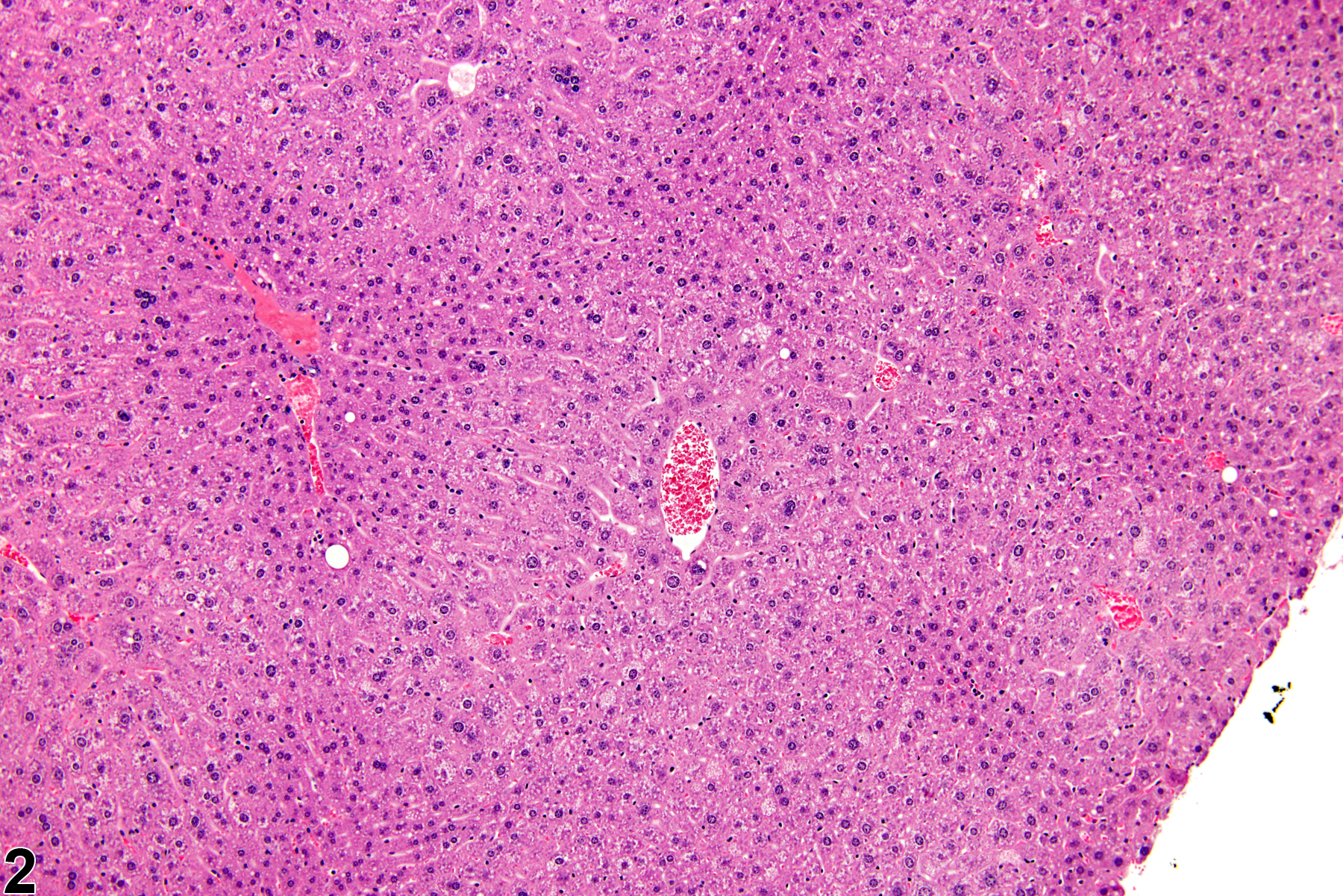
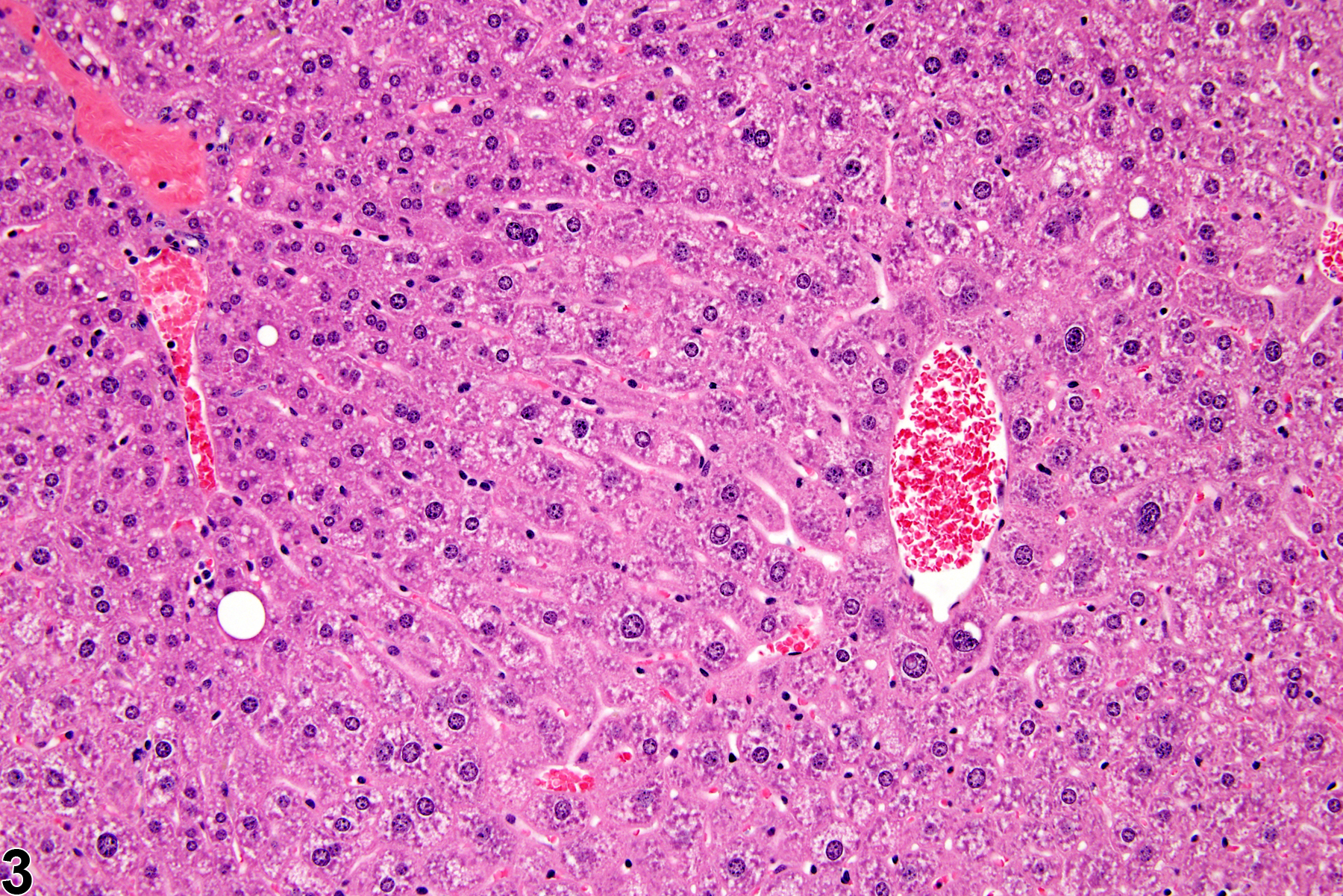
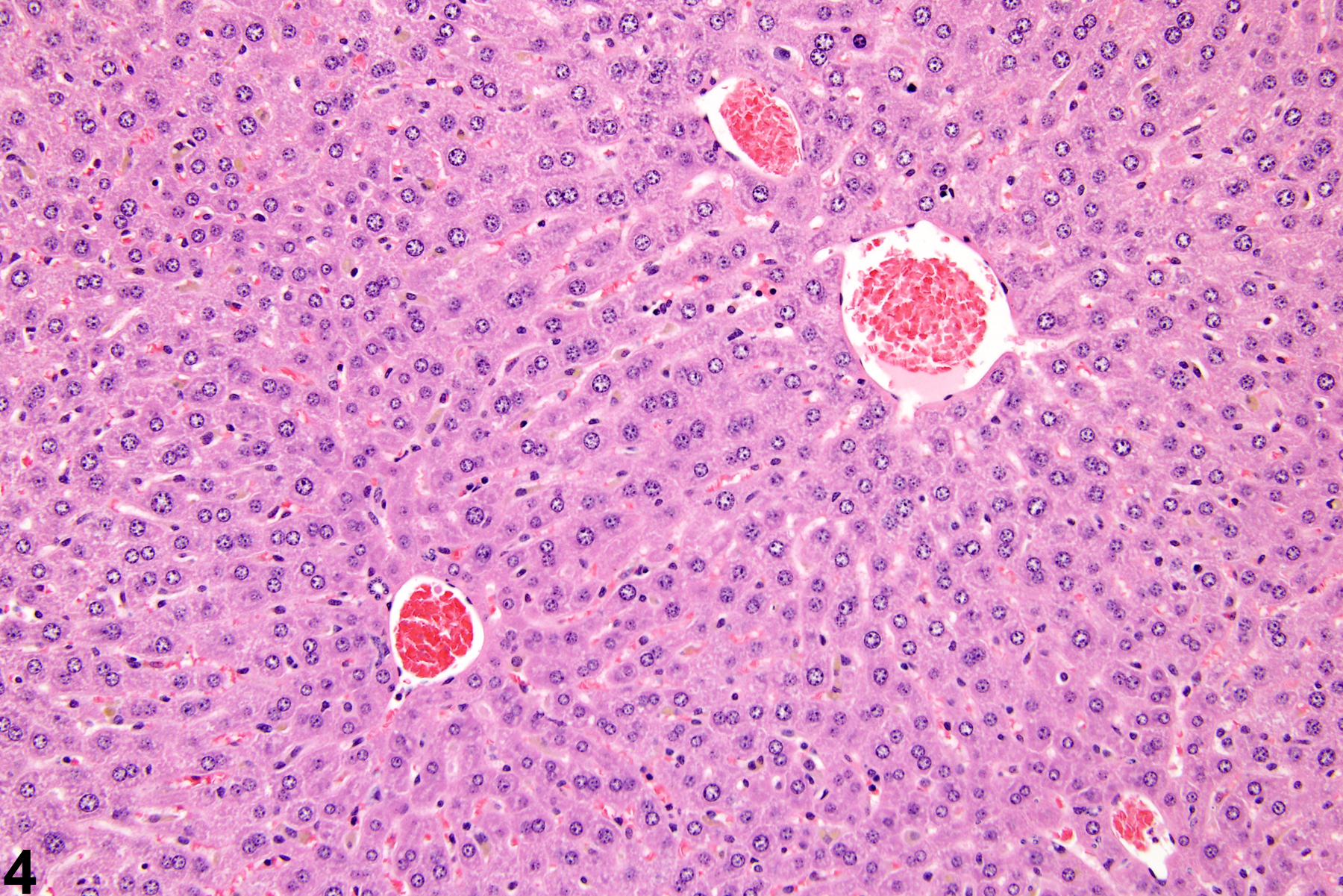
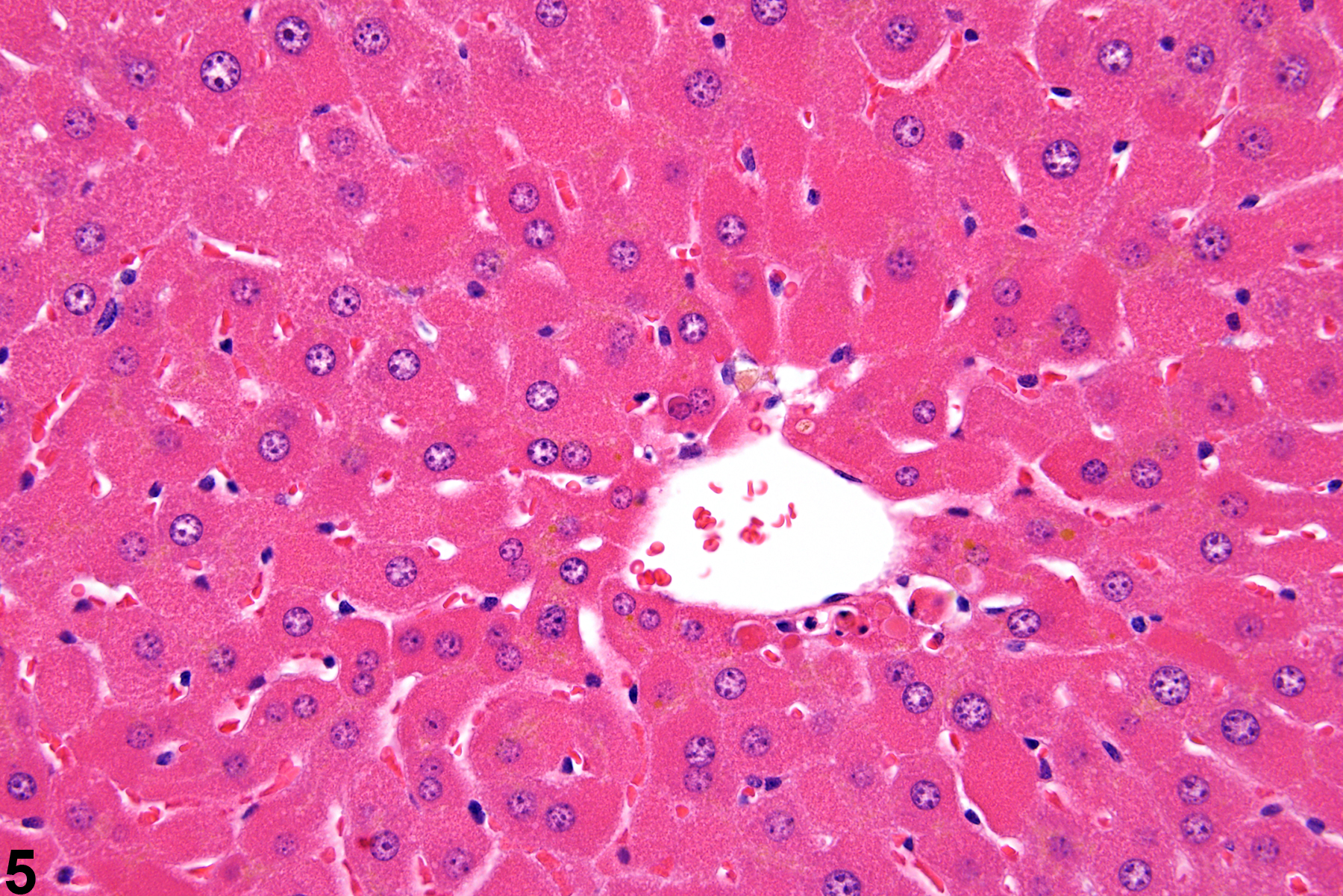
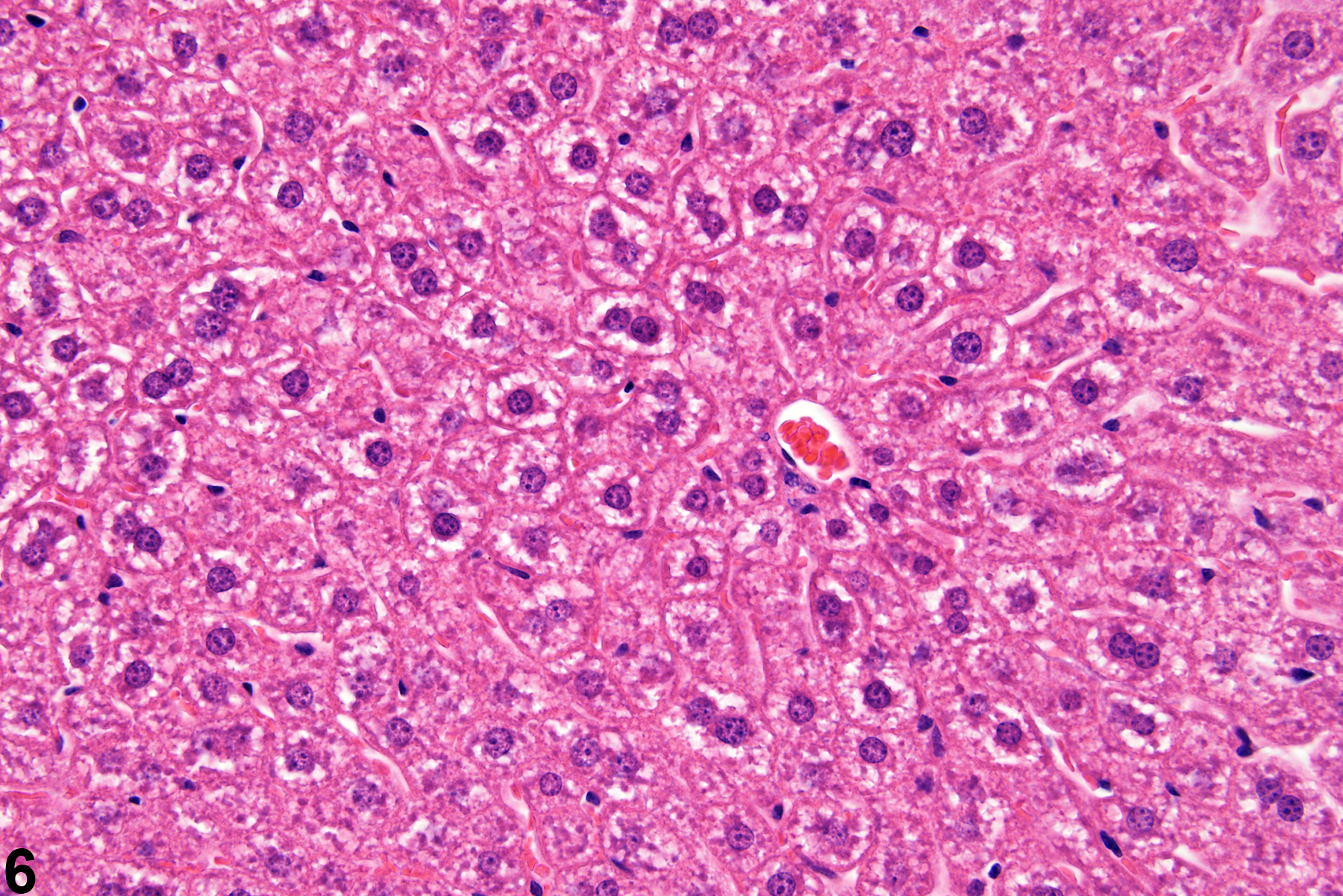
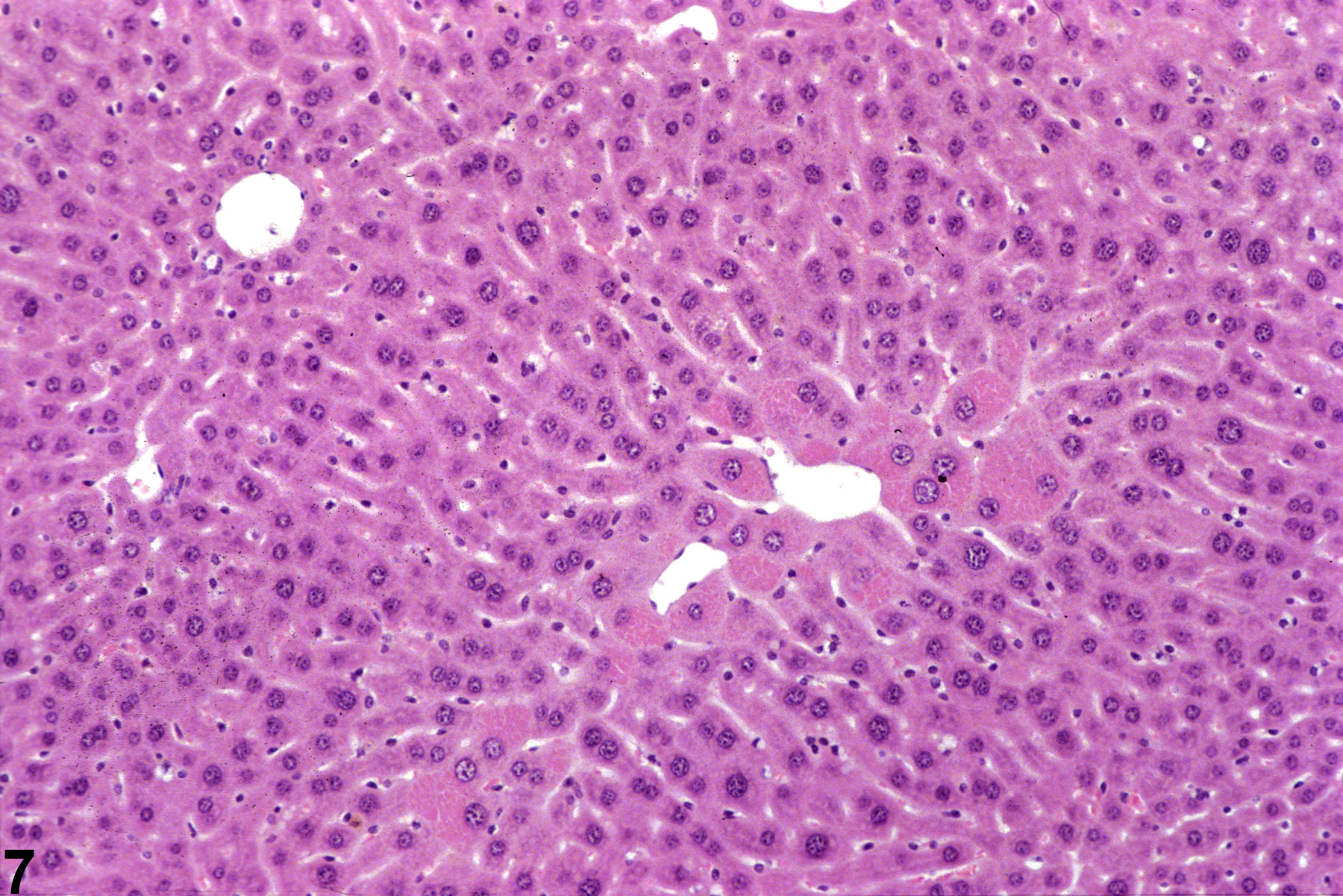
Hepatocyte hypertrophy is commonly associated with microsomal enzyme induction secondary to exposure to certain xenobiotics (Figure 1, Figure 2, and Figure 3). It most frequently affects centrilobular hepatocytes, depending upon the xenobiotic and the dose administered, although the hypertrophy can extend into the middle of the hepatic lobule or become panlobular. It is also possible for periportal hepatocytes to be primarily affected, though care must be taken not to confuse periportal hypertrophy with processes that result in shrinkage of centrilobular hepatocytes (e.g., glycogen depletion). Figure 4 is a normal control liver from the same study for comparison with Figure 3. Depending upon the specific xenobiotic responsible, hypertrophic hepatocyte cytoplasm may have a pale, ground glass appearance or be granular and intensely eosinophilic, especially following exposure to peroxisome proliferators (Figure 5). Figure 6 is a concurrent control liver for comparison with Figure 5. Marked hypertrophy with associated karyomegaly and multinucleated hepatocytes may be seen with chronic exposure to some nongenotoxic hepatic toxicants.
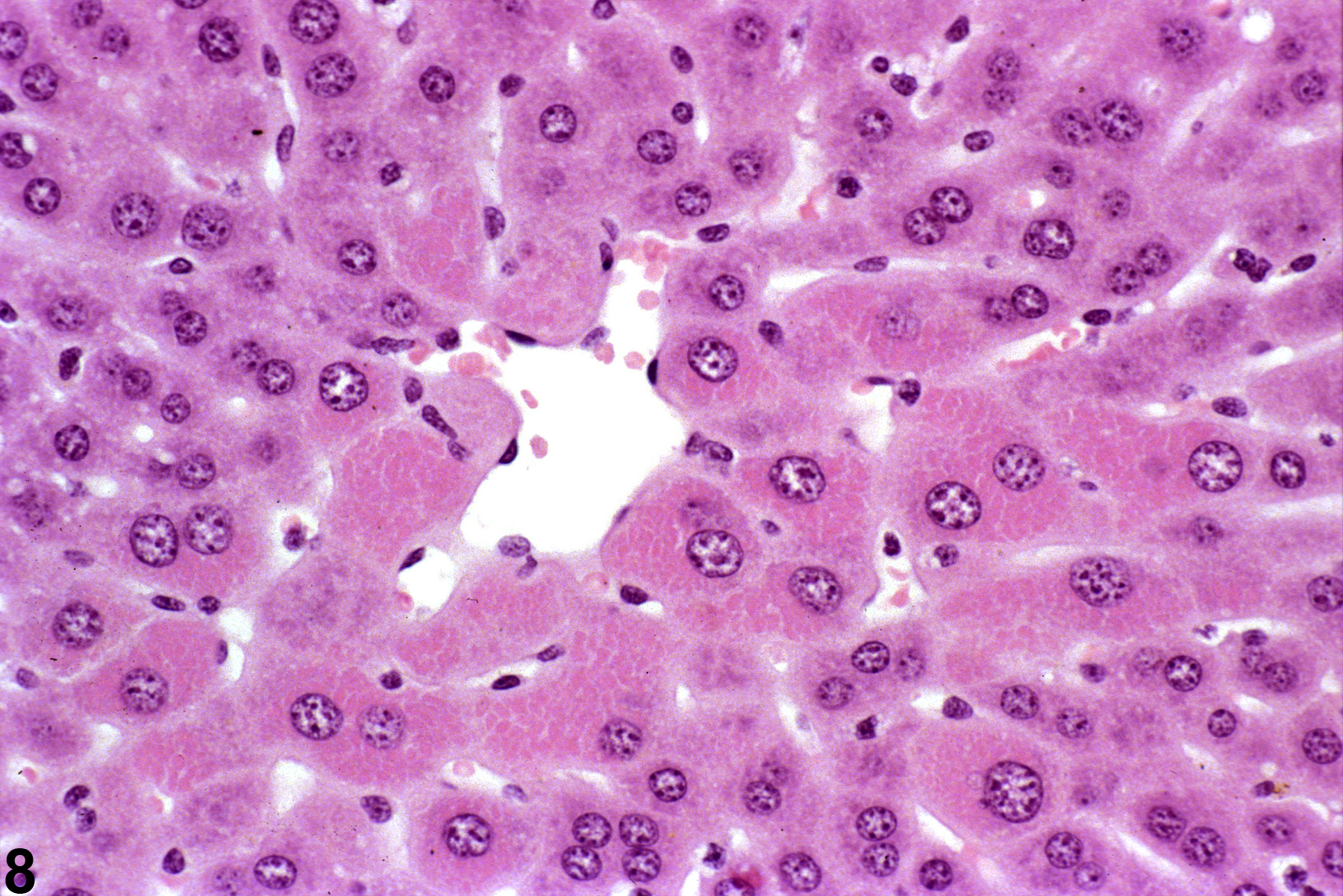
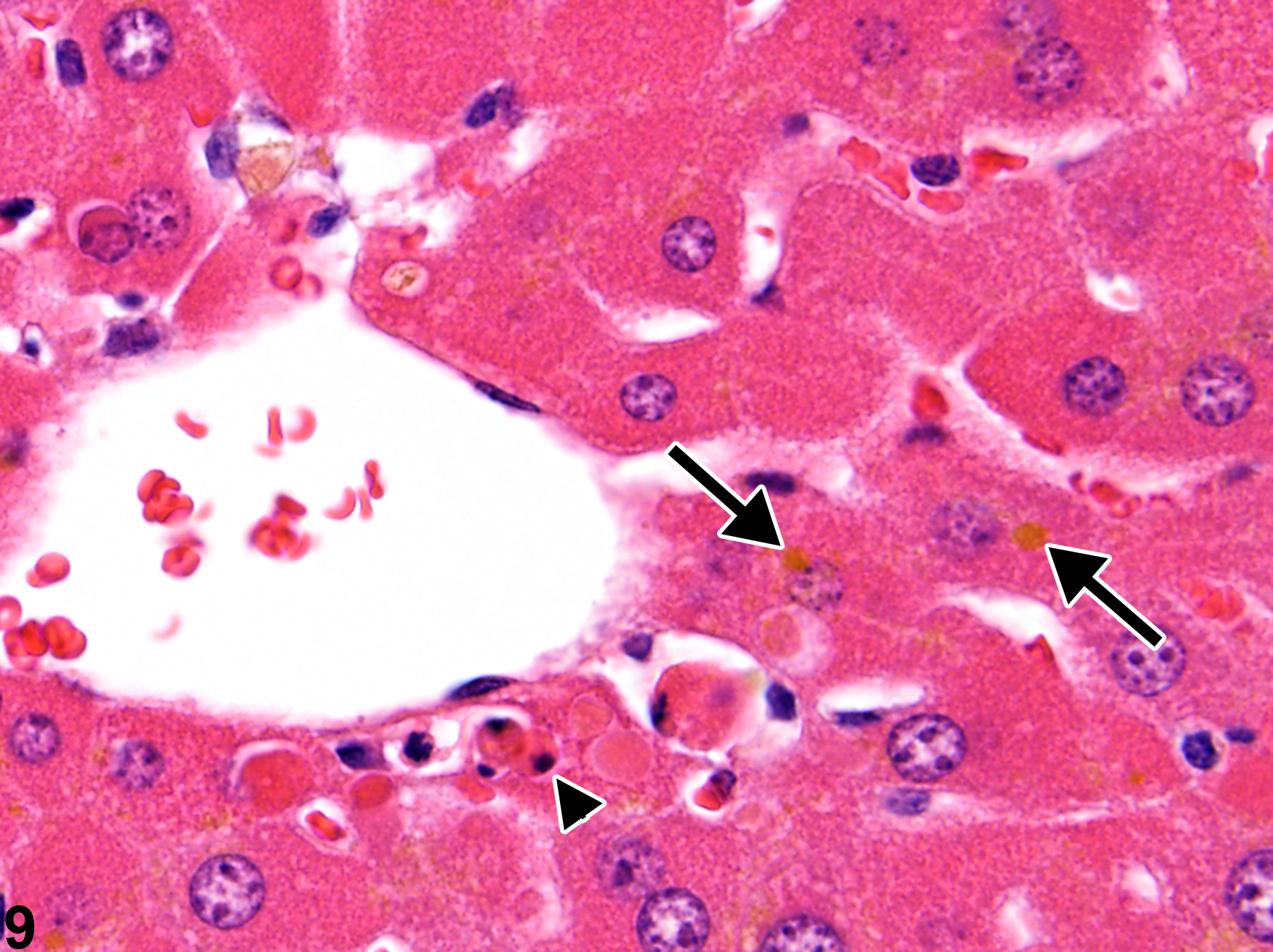
A narrow centrilobular zone of hepatocyte hypertrophy is present in Figure 7 (higher magnification in Figure 8). The finely granular eosinophilic cytoplasm in the enlarged hepatocytes is similar to that seen with peroxisome proliferation, but in this case the cytologic alteration responsible for the hepatocyte hypertrophy was considered hyaline change. In extreme forms, hyaline change can form distinct cytoplasmic inclusions, sometimes referred to as Mallory bodies. Figure 9 is a high magnification of the central vein area of Figure 5 and shows apoptosis of hepatocytes (arrowhead) and retention of bile pigment (arrows). Hepatocyte degeneration and cell death can occur because of reduced blood flow secondary to sinusoidal compression by enlarged hepatocytes.
Cattley RC, Popp JA. 2002. Liver. In: Handbook of Toxicologic Pathology (Haschek WM, Rousseaux CG, Wallig MA, eds). Academic Press, San Diego, 2:187-225.
Eustis SL, Boorman GA, Harada T, Popp JA. 1990. Liver. In: Pathology of the Fischer Rat (Boorman GA, Eustis SL, Elwell MR, Montgomery CA, MacKenzie WF, eds). Academic Press, San Diego, 71-94.
Evans JG, Lake BG. 1998. The digestive system II. Hepatobiliary system. In: Target Organ Pathology (Turton J, Hooson J, eds). Taylor and Francis, London, 61-98.
Greaves P. 2007. Histopathology of Preclinical Toxicity Studies: Interpretation and Relevance in Drug Safety Evaluation, 3rd ed. Elsevier, Amsterdam.
Abstract: http://www.sciencedirect.com/science/book/9780444527714Harada T, Enomoto A, Boorman GA, Maronpot RR. 1999. Liver and gallbladder. In: Pathology of the Mouse: Reference and Atlas (Maronpot RR, Boorman GA, Gaul BW, eds). Cache River Press, Vienna, IL, 119-183.
Abstract: http://www.cacheriverpress.com/books/pathmouse.htmHardisty JF, Brix AE. 2005. Comparative hepatic toxicity: Prechronic/chronic liver toxicity in rodents. Toxicol Pathol 33:35-40.
Full Text: http://tpx.sagepub.com/content/33/1/35.full.pdfHaschek WM, Rousseaux CG, Wallig MA. 2010. Fundamentals of Toxicologic Pathology, 2nd ed. Academic Press, San Diego, 197-235.
Maronpot RR, Yoshizawa K, Nyska A, Harada T, Flake G, Mueller G, Singh B, Ward JM. 2010. Hepatic enzyme induction: Histopathology. Toxicol Pathol 38:776-795.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/20585142National Toxicology Program. 1993. NTP TR-413. Toxicology and Carcinogenesis Studies of Ethylene Glycol (CAS No. 107-21-1) in B6C3F1 Mice (Feed Studies). NTP, Research Triangle Park, NC.
Full Text: https://ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr413.pdfNational Toxicology Program. 2012. NTP TR-571. Toxicology and Carcinogenesis Studies of Kava Kava Extract (CAS No. 9000-38-8) in F344/N Rats and B6C3F1 Mice (Gavage Studies). NTP, Research Triangle Park, NC.
Full Text: https://ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr571.pdfThoolen B, Maronpot RR, Harada T, Nyska A, Rousseaux C, Nolte T, Malarkey D, Kaufmann W, Kutter K, Deschl U, Nakae D, Gregson R, Winlove M, Brix A, Singl B, Belpoggi F, Ward JM. 2010. Hepatobiliary lesion nomenclature and diagnostic criteria for lesions in rats and mice (INHAND). Toxicol Pathol 38:5S-81S.
Full Text: http://tpx.sagepub.com/content/38/7_suppl/5S.full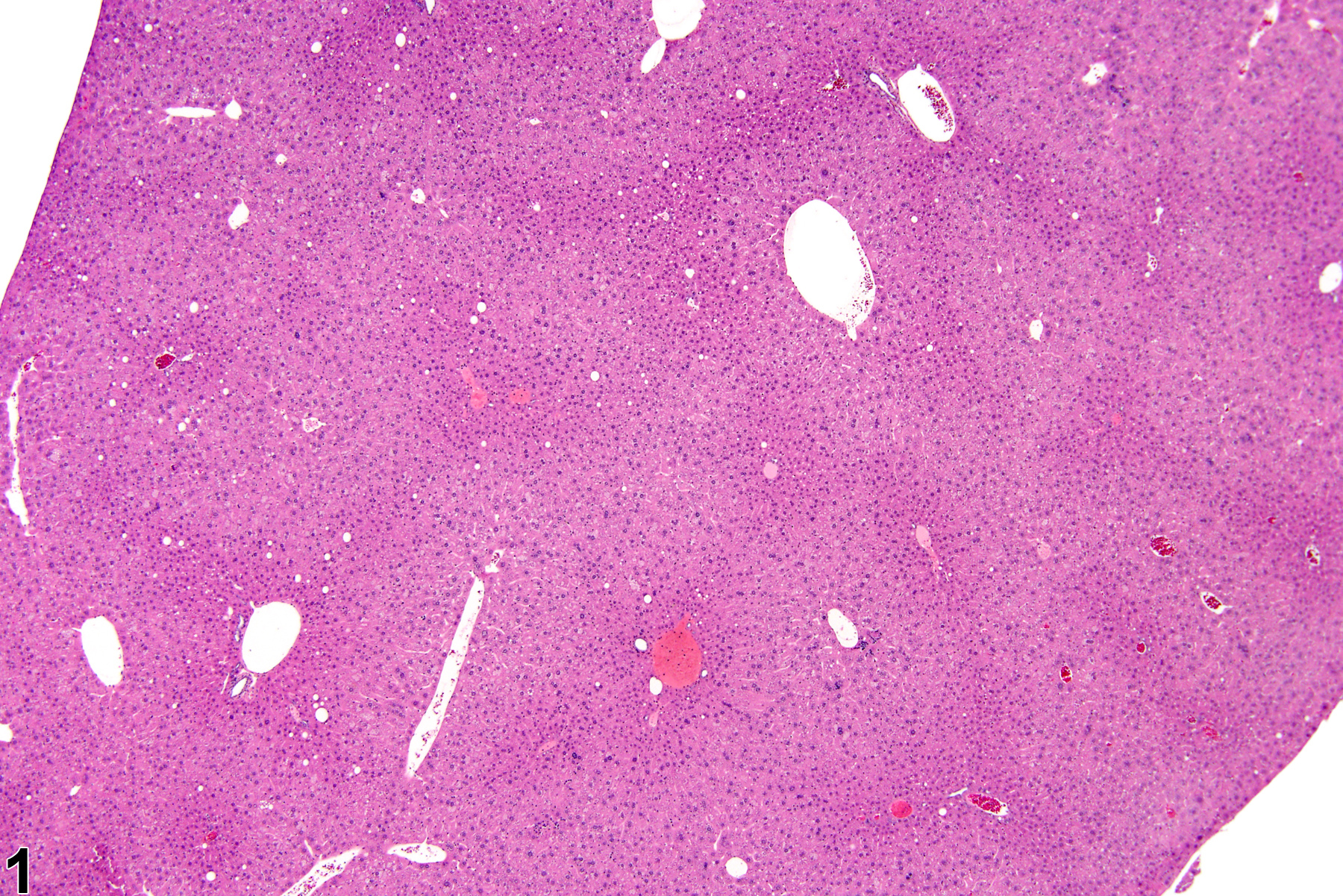
Hepatocyte hypertrophy in a male B6C3F1 mouse from a chronic study.