Musculoskeletal System
Bone - Increased Bone
Narrative
Increased bone is defined as excessive growth of normal bone. The lesion is characterized by increased amounts of mature cortical (compact) and cancellous (trabecular, spongy) bone formation, resulting in generalized thickening of the cortices and trabeculae with diminution of marrow spaces (Figure 1 and Figure 2). Increased bone results from either decreased resorption of bone by osteoclasts or increased bone formation by osteoblasts. The current terminology used to describe the nonneoplastic proliferation of bone is often confusing and inconsistent, with the same lesion being described as hyperostosis, bony hyperplasia, osteopetrosis, osterosclerosis, reactive bone, bony proliferation, and exostoses. The use of the term "increased bone" is recommended to prevent redundancy in the nomenclature and confusion in interpretation of study findings.
While rarely observed as a treatment-related effect, an increased amount of bone is an occasional background lesion seen in aged F344 rats, with females more commonly affected than males. This lesion more commonly affects the cranium than the long bones and results in diminished or nonexistent marrow cavities and markedly thickened and dense cranial and nasal turbinate bones. In rats, estrogen toxicity results in decreased bone resorption from metaphyseal trabeculae, leading to densely thickened medullary bone and overall shortened length of the bone. Increased bone in the nasal cavity turbinates has been reported to occur in long-term studies involving chronic inflammation of the nasal cavity (Figure 3 and Figure 4).
The term "fibrous osteodystrophy" is incorrectly used when there are small amounts of fibrous connective tissue deposited along trabeculae of hyperostotic bone. Fibrous osteodystrophy is associated with loss of mature bone and thus should not be diagnosed when there is increased deposition of bone. Rather, this fibrotic component is a normal constituent of an increase in bone in many cases.
While it is usually a background lesion, increased bone should be diagnosed and given a severity grade.
Chhabra RS, Huff JE, Haseman J, Hall A, Baskin G, Cowan M. 1988. Inhibition of some spontaneous tumors by 4-hexylresorcinol in F344/N rats and B6C3F1 mice. Fundam Appl Toxicol 1:685-690.
Abstract: http://www.ncbi.nlm.nih.gov/pubmed/3229592Dodd DC, Port CD. 1987. Hyperostosis of the marrow cavity caused by misoprostol in CD-1 strain mice. Vet Pathol 24:545-548.
Abstract: http://www.ncbi.nlm.nih.gov/pubmed/3137716Leininger JR, Riley MGI. 1990. Bones, joints, and synovia. In: Pathology of the Fischer Rat: Reference and Atlas (Boorman G, Eustis SL, Elwell MR, Montgomery CA, MacKenzie WF, eds). Academic Press, San Diego, 209-226.
Long PH, Leininger JR. 1999. Bones, joints, and synovia. In: Pathology of the Mouse (Maronpot R, Boorman G, Gaul BW, eds). Cache River Press, St Louis, 645-678.
Jee WS, Ueno K, Deng YP, Woodbury DM. 1985. The effects of prostaglandin E2 in growing rats: Increased metaphyseal hard tissue and cortico-endosteal bone formation. Calfic Tissue Int 37:148-157.
Abstract: http://www.ncbi.nlm.nih.gov/pubmed/3924371Maurer JK, Cheng MC, Boysen BG, Squire RA, Strandberg JD, Weisbrode SE, Seymour JL, Anderson RL. 1993. Confounded carcinogenicity study of sodium fluoride in CD-1 mice. Regul Toxicol Pharmacol 18:154-168.
Abstract: http://www.ncbi.nlm.nih.gov/pubmed/8278638Moutier R, Toyama K, Cotton WR, Gaines JF. 1976. Three recessive genes for congenital osteopetrosis in the Norway rat. J Hered 67:189-190.
Abstract: http://www.ncbi.nlm.nih.gov/pubmed/780413Raisz LG, Kream BE. 1983. Regulation of bone formation. N Engl J Med 309:29-35, 83-89.
Silberberg M, Silberberg R. 1970. Age-linked modification of the effect of estrogen on joints and cortical bone of female mice. Gerontologica 16:201-211.
Simmons DJ. 1966. Collagen formation and endochondral ossification in estrogen treated mice. Proc Soc Exp Biol Med 121:1165-1168.
Abstract: http://ebm.sagepub.com/content/121/4/1165.abstract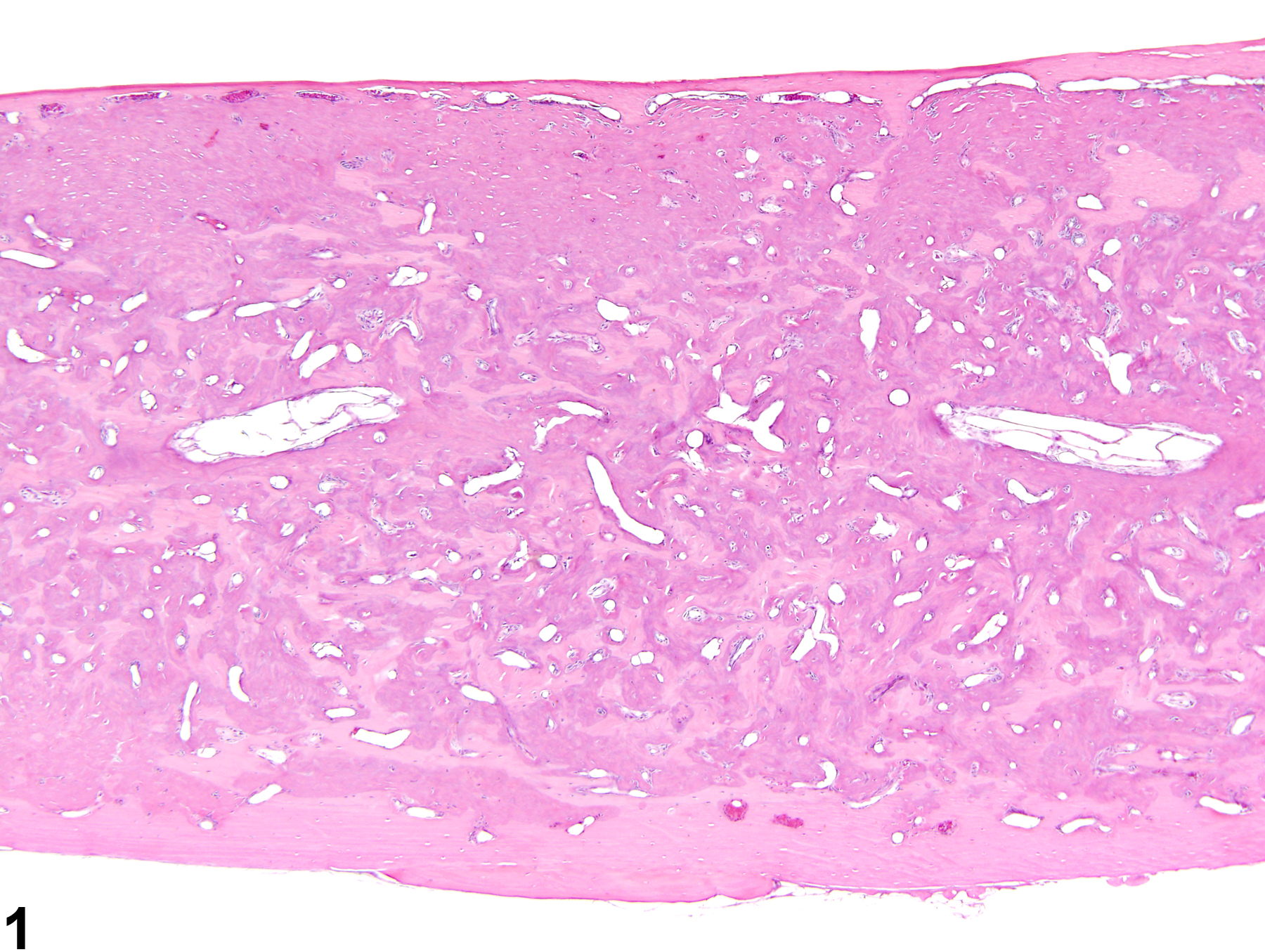
Bone - Increased bone in a female B6C3F1/N mouse from a chronic study. The medulla of this femur has been obliterated by increased amounts of bone.





