Cardiovascular System
Blood Vessel - Mineral
Narrative
Mineral appears as fine basophilic granules or crystalline material that may be located within the tunica media or tunica intima of arteries (Figure 1, Figure 2, Figure 3, and Figure 4) or rarely within veins. With time, lesions may progress to form large, amorphous, basophilic concretions that distort the vascular wall. Mineral can be a feature of the advancement of atherosclerotic lesions and may progress to form cartilage or bone. Special stains such as von Kossa or alizarin red are often employed to detect these calcium salts.
Mineral may develop secondary to areas of necrosis (dystrophic) or in conjunction with renal disease (metastatic). In rats, mineral is commonly seen secondary to severe renal disease (chronic progressive nephropathy) and hyperparathyroidism but may also be spontaneous or toxin related. Mineral in the thoracic and abdominal aortas is observed in control and treated F344 rats with severe spontaneous and treatment-exacerbated chronic nephropathy, parathyroid hyperplasia, and fibrous osteodystrophy. Mineral without secondary renal hyperparathyroidism may also be seen in the femoral, mesenteric, spermatic, and pulmonary arteries, as well as in medium-sized arteries in the kidney, testis, pancreas, stomach, heart, tongue, lung, spleen, salivary gland, and skeletal muscle. Mineral in small vessels of the thalamus of the brain is a relatively common background lesion in both sexes of B6C3F1/N mice from chronic studies.
Many models of arterial calcification exist in both rat and mouse, with most models generated through knockout technology involving, for example, Smad6, matrix Gla protein (MGP), osteoprotegrin, fibrillin-1, and carbonic anhydrase isoenzyme II. The specific vascular distribution, morphologic characteristics, mortality, and sex predilection are highly variable and depend on the strain used and the specific gene eliminated. Generally, the MGP-null mouse has produced the most extensive arterial calcification thus far. Additionally, a genetic predisposition to vascular calcification has been shown in some mouse strains. DBA/2J mice are highly susceptible to coronary and aortic calcification, even when maintained on a nonatherogenic diet. C3H/HeJ mice show susceptibility to calcification of the coronary arteries.
Administration of a variety of stimulants, such as warfarin, vitamin D, and nicotine, have been used to induce arterial calcification in a variety of different animals. Exposing rats to warfarin resulted in mineralization of the aortic media, as well as the media of smaller arteries; however, combining warfarin and vitamin D produced even greater arterial calcification.
Mineral in blood vessels should be diagnosed and graded based on the extent of deposition. The affected organ should be used as the site for the diagnosis, with the specific type of vessel (e.g., artery or vein) indicated as a site modifier. If the type of blood vessel cannot be determined, the site modifier "blood vessel" may be used. Lesions in protocol-required great vessels, such as aorta, should be recorded with the blood vessel as the site (e.g., Aorta - Mineral). The location of the mineral within the vessel, either media or intima, should be discussed in the pathology narrative, as it may provide clues to the underlying pathology. If mineral is a component of another primary process such as atherosclerosis or necrosis, it should not be diagnosed separately, unless warranted by severity, though it may be mentioned in the narrative. Any suspected correlation with chronic progressive nephropathy (metastatic mineralization) should also be discussed in the narrative.
Brown AP, Courtney CL, King LM, Groom SC, Graziano MJ. 2005. Cartilage dysplasia and tissue mineralization in the rat following administration of a FGF receptor tyrosine kinase inhibitor. Toxicol Pathol 33:449-455.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/16036862Dungworth DL, Ernst H, Nolte T, Mohr U. 1992. Nonneoplastic lesions in the lungs. In: Pathobiology of the Aging Rat (Mohr U, Capen CC, Dungworth DL, eds). ILSI Press, Washington, DC, 143-160.
Mitsumori K. 1990. Blood and lymphatic vessels. In: Pathology of the Fischer Rat: Reference and Atlas (Boorman GA, Eustis SL, Elwell MR, Montgomery CA, MacKenzie WF, eds). Academic Press, San Diego, CA, 473-483.
Morony S, Sage AP, Corbin T, Lu J, Tintut Y, Demer LL. 2012. Enhanced mineralization potential of vascular cells from SM22α-Rankl (tg) mice. Calcif Tissue Int 91(6):379-386.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/23052229Wallin R, Wajih N, Grenwood GT, Sane DC. 2001. Arterial calcification: A review of mechanisms, animal models, and the prospects for therapy. Med Res Rev 21(4):274-301.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/11410932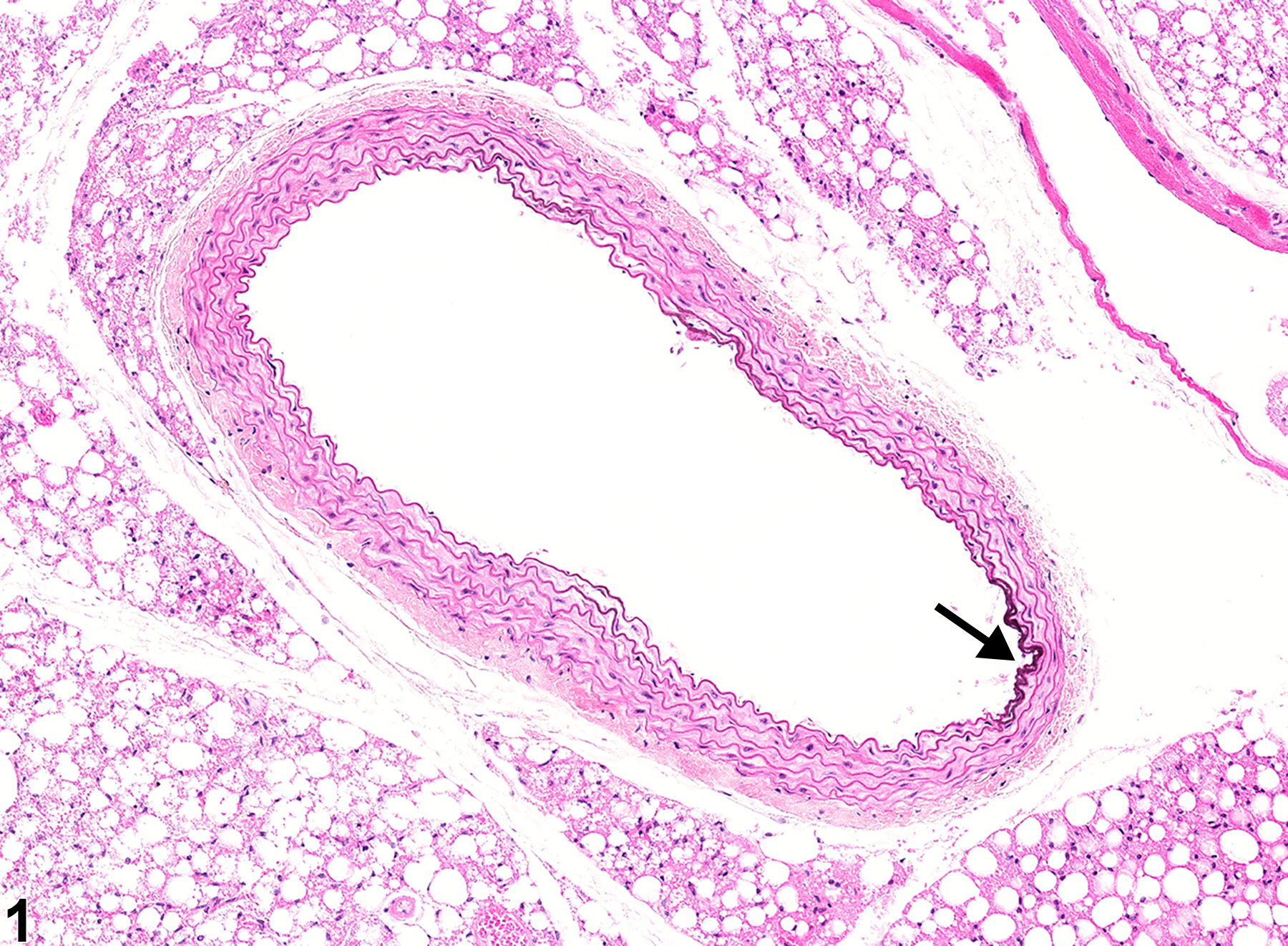
Aorta - Mineral in a male B6C3F1/N mouse from a chronic study. There is mineral in a segment of the aortic intima (arrow).





