Endocrine System
Adrenal Gland, Cortex - Degeneration, Cystic
Narrative
Early stages of cystic degeneration are characterized by small, noncompressive foci of widened cortical sinusoids and dilated blood vessels in the zona fasciculata; cortical cells in the intervening cords can be vacuolated or slightly flattened. As the lesion progresses, affected areas enlarge and can compress adjacent parenchyma (Figure 3 and Figure 4). The sinusoidal and vascular ectasia progressively becomes more extensive, resulting in large, often confluent, cyst-like cavitations (Figure 1, Figure 2, Figure 3, Figure 4, and Figure 5) that are filled with proteinaceous fluid, red blood cells, and/or fibrinous thrombi but lack the epithelial lining characteristic of true cysts. Clusters of vacuolated or swollen cortical cells can be scattered among the dilated spaces. Areas of cystic degeneration can also be features of large cortical hyperplastic or hypertrophic foci or of cortical neoplasms; however, in cystic degeneration the number of cells is decreased compared with normal cortex.
Brix AE, Nyska A, Haseman JK, Sells DM, Jokinen MO, Walker NJ. 2005. Incidences of selected lesions in control female Harlan Sprague-Dawley rats from two-year studies performed by the National Toxicology Program. Toxicol Pathol 33:477-483.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/16036865Dohm G, Hohbach C, Mäusle E, Scherr O, Ueberberg H. 1981. Peliosis of the female adrenal cortex of aging rats. Virchows Arch B (Cell Pathol) 36:195-206.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/6116334Elmore SA, Berridge BR, Boyle MC, Cora MC, Hoenerhoff MJ, Kooistra L, Laast VA, Morrison JP, Rao D, Rinke M, Yoshizawa K. 2013. Proceedings of the 2012 National Toxicology Program Satellite Symposium. Toxicol Pathol 41:151-180.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/23262640Frith CH, Botts S, Jokinen MP, Eighmy JJ, Hailey JR, Morgan SJ, Chandra M. 2000. Non-proliferative lesions of the endocrine system in rats, E-1. In: Guides for Toxicologic Pathology. STP/ARP/AFIP, Washington, DC.
Full Text: https://www.toxpath.org/docs/SSNDC/EndocrineNonprolifRat.pdfHamlin MH, Banas DA. 1990. Adrenal gland. In: Pathology of the Fischer Rat: Reference and Atlas (Boorman GA, Eustis SL, Elwell MR, Montgomery CA, MacKenzie WF, eds). Academic Press, San Diego, 501-518.
Abstract: https://www.ncbi.nlm.nih.gov/nlmcatalog/9002563Imazawa T, Nishikawa A, Todate A, Furukawa F, Onodera H, Mitsumori K, Hirose M, Hayashi Y. 2000. Dual effects of prolonged ACTH stimulation on 4-hydroxyaminoquinoline 1-oxide-induced adrenocortical lesions in rats. Toxicol Pathol 28:535-539.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/10930039McMartin DL, Sahota PS, Gunson DE, Han Hsu H, Spaet RH. 1992. Neoplasms and related proliferative lesions in control Sprague-Dawley rats from carcinogenicity studies. Historical data and diagnostic considerations. Toxicol Pathol 20:212-225.
Abstract: https://www.ncbi.nlm.nih.gov/pubmed/1475582National Toxicology Program. 2006. NTP TR-525. Toxicology and Carcinogenesis Studies of 2,3,4,7,8-Pentachlorodibenzofuran (PeCDF) (CAS No. 57117-31-4) in Female Harlan Sprague-Dawley Rats (Gavage Studies). NTP, Research Triangle Park, NC.
Abstract: https://ntp.niehs.nih.gov/go/9304National Toxicology Program. 2010. NTP TR-559. Toxicology and Carcinogenesis Studies of 2,3',4,4',5-Pentachlorobiphenyl (PCB 118) (CAS No. 31508-00-6) in Female Harlan Sprague-Dawley Rats (Gavage Studies). NTP, Research Triangle Park, NC.
Abstract: https://ntp.niehs.nih.gov/go/33539Nyska A, Maronpot RR. 1990. Adrenal gland. In: Pathology of the Mouse: Reference and Atlas (Maronpot RR, Boorman GA, Gaul BW, eds). Cache River Press, Vienna, IL, 509-536.
Abstract: http://www.cacheriverpress.com/books/pathmouse.htmTucker MJ. 1997. Diseases of the Wistar rat. In: The Endocrine System. Taylor & Francis, London: 183-215.
Abstract: http://www.crcpress.com/product/isbn/9780748405213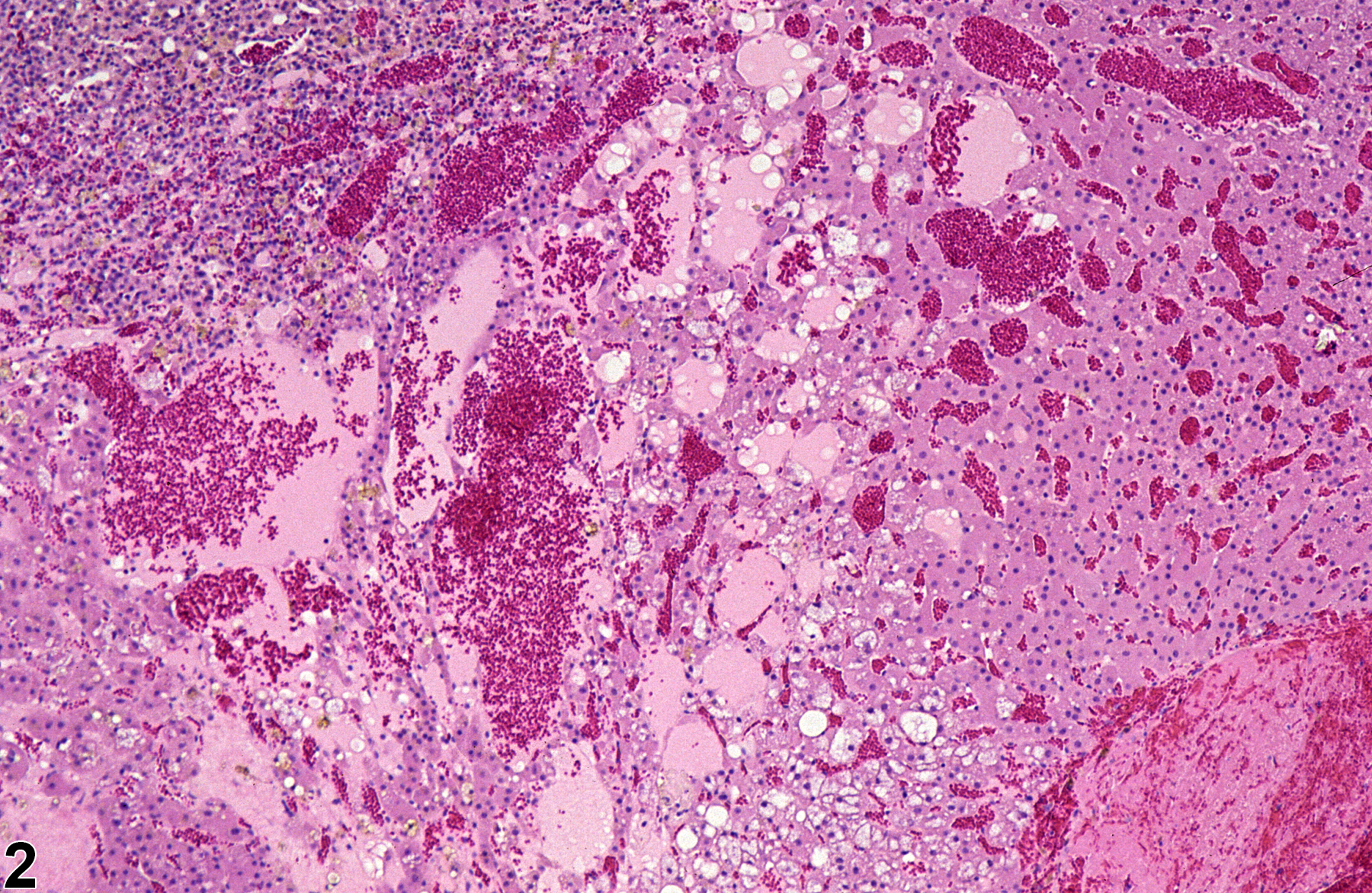
Adrenal gland, Cortex - Degeneration, Cystic in a female Sprague-Dawley rat from a chronic study (higher magnification of Figure 1). Cystic degeneration is characterized by dilated spaces filled with proteinaceous fluid and/or blood and scattered vacuolated cortical cells.






